Indigenous Australians living in remote areas are twice as likely to have diabetes than Indigenous Australians living in non-remote areas1. The management of diabetes in remote locations has been historically difficult due to the limited access to resources at the health service level2,3. In Australia's Northern Territory (NT), remote health centres are, on average, 275 km from the nearest laboratory (range 100-700 km), making timely access to pathology results and subsequent follow-up of patients for treatment very difficult4-7. Point-of-care testing (POCT) offers a practical solution for pathology service provision in such remote communities. Through the national Quality Assurance in Aboriginal and Torres Strait Islander Medical Services (QAAMS) Program8-11, POCT for haemoglobin A1c (HbA1c) has been conducted in remote NT health centres for diabetes management of Indigenous patients since 2008. Here we present the results of a clinical audit comparing the timeliness of HbA1c testing before and after POCT was introduced into these remote health centres.
Thirty remote health centres in the NT currently participate in the QAAMS Program through a partnership between the NT Department of Health and the Flinders University International Centre for Point-of-Care Testing. HbA1c POCT is performed on-site in the remote health centres using the DCA analyser (Siemens Healthcare Diagnostics; http://www.healthcare.siemens.com.au/point-of-care/diabetes/dca-vantage-analyzer), which uses 1 µL of capillary whole blood and provides a result in 6 min. Operators performing the tests (remote area nurses and Aboriginal Health Practitioners) undergo training and competency certification through the QAAMS Program.
An audit of the number of HbA1c results performed by POCT on Indigenous diabetes patients from July 2008 to April 2011 was undertaken by accessing the NT's Primary Care Clinical Information System (PCIS).
Patients who had three or more HbA1c results performed by POCT across this period were assessed to determine their overall change in glycaemic control. A subgroup of these patients (who exhibited a decrease in HbA1c levels of more than 1.5%) underwent a more detailed clinical audit to compare selected parameters across a 15-month period before POCT was introduced (when HbA1c testing was performed by the nearest local laboratory) and the 15-month period after POCT had been introduced. The parameters examined in this 'before and after' dataset included:
- change in glycaemic control (mean change in HbA1c ± standard deviation)
- turnaround time of result reporting (calculated by determining the time taken, in hours, between collecting the blood sample from the patient and the result being received from the laboratory or POCT device)
- turnaround time for follow-up consultation and management of the patient (calculated by determining the time taken, in days/hours, between collecting the blood sample from the patient and the treating medical practitioner consulting with patient about their laboratory or POCT HbA1c result and initiating management
- number of HbA1c tests performed per patient.
Paired t-tests were used to determine the statistical significance of change in glycaemic control and the number of tests performed per patient before and after POCT was introduced.
Ethics approval
Ethics approval to source this data was obtained through the Menzies School of Health Research (registration number: QAAR-2012-1823) in the NT. Confidentiality of patient data was assured because only de-identified patient information was analysed for the audit. Only pathology results collected for routine patient care were analysed in the audit. The audit did not result in changes to patient's routine clinical care. The audit formed part of the Northern Territory POCT Management Committee's charter to investigate the clinical effectiveness of POCT being undertaken as part of the program on behalf of the Northern Territory Government's Department of Health.
A total of 907 Indigenous diabetes patients had 1594 HbA1c tests performed by POCT at the participating remote NT health centres from July 2008 to April 2011. A total of 181 patients (20%) had three or more HbA1c POCT performed (range 3-8 tests) during this period, with the mean HbA1c in this group falling significantly from 9.2% (77 mmol/mol) ± 2.1 (at their first POCT measurement) to 8.8% (73 mmol/mol) ± 2.2 (at their most recent measurement) (paired t-test p=0.013).
Forty of the 181 patients showed a greater than 1.5% reduction in HbA1c from their first to their most recent POCT over a mean period of 15 ± 6 months.
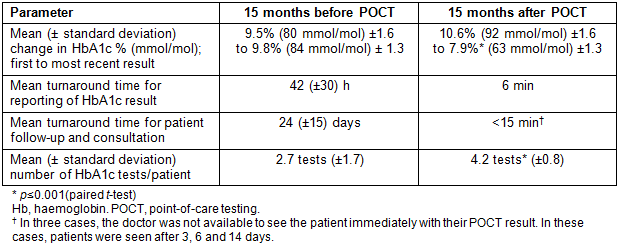
Table 1 compares the change in glycaemic control, turnaround times for result reporting and patient follow-up/management and HbA1c testing frequency for this subset of 40 patients across an equivalent time period (mean 15 ± 6 months) before and after the introduction of POCT.
Discussion
The mean HbA1c within this group decreased significantly by 2.7% (paired t-test p≤0.001) over the 15-month period post-POCT, while there was minimal change in glycaemic control (+0.3%) when the local laboratory was used to monitor HbA1c prior to the introduction of POCT.
The mean turnaround time from sample collection to receipt of result was 2.3 days when the laboratory was performing HbA1c testing, but was just 6 minutes (that is, the time taken for the result to be available on the DCA device) after POCT was introduced.
Importantly, when the laboratory performed the HbA1c and the patient was required to return to the service for a follow-up visit, the mean time to consult with the doctor was 24 days in this remote setting, compared to an immediate consultation with the doctor during the same visit post-POCT.
The mean number of HbA1c tests per patient was higher (by 1.5) following the introduction of POCT (p≤0.001), being more consistent with the clinical recommendations for optimal frequency of HbA1c testing for poorly controlled Indigenous diabetes patients (four tests per year)12.
The provision of pathology laboratory services in remote locations such as the NT is severely compromised due to the region's geographic isolation and long distances between health centres and local laboratories, and to difficulties in being able to recall patients living in remote communities for follow-up consultation and actioning of pathology results. For these reasons, the Northern Territory Government decided in 2008 to invest in the opportunity to integrate quality-assured POCT into pathology service provision for its remote health centres. Many remote communities now have access to POCT for both chronic disease care (through the QAAMS Program) and acute clinical care13,14 (using the i-STAT POCT device (Abbott Point of Care, Melbourne, Australia)).
POCT provides many advantages as a mode of pathology service provision for Indigenous patients, including convenience and accessibility, increased sense of ownership of their blood samples, motivation to take control of their own health and an improved relationship with their doctor10. For the treating medical practitioner, the ability to see the patient and enact changes of management/treatment 'on the spot' facilitates improved clinical care.
In previous prospective longitudinal studies relating to POCT in Indigenous settings, our work has shown that glycaemic control for Indigenous diabetes has improved following the introduction of POCT into rural and remote communities10,15. In this study, we have compared the clinical and operational efficiency of POCT versus laboratory testing over an equivalent time period (15 months) using a 'before and after' study design across remote health centres in the NT. This study design has enabled quantitation of the delays experienced in providing laboratory services and clinical actioning of laboratory results in the most remote and challenging health service setting in Australia. At the same time, this study reinforces the clinical and cultural effectiveness of POCT and its critical role in assisting to improve diabetes management in Indigenous Australians.
Acknowledgements
The authors would like to acknowledge the continuing contribution of the following members of the Northern Territory POCT Management Committee to the program's success and sustainability: Dr Vinod Daniel, Malcolm Auld, Amanda Lingwood, John Louden, Janet Rigby and Beryl Mazzachi.
The QAAMS Program is funded by the Australian Government's Department of Health.
References
1. Minges KE, Zimmet P, Magliano DJ, Dunstan DW, Brown A, Shaw JE. Diabetes prevalence and determinants in Indigenous Australian populations: a systematic review. Diabetes Research and Clinical Practice 2011; 93: 139-149.
2. Azzopardi P, Brown A, Zimmet P, Fahy R, Dent G, Kelly M, et al. Type 2 diabetes in young Indigenous Australians in rural and remote areas: diagnosis, screening, management and prevention. Medical Journal of Australia 2012; 197(1): 32.
3. Bailie R, Si D, Dowden M, O'Donoghue L, Connors C, Robinson G, et al. Improving organisational systems for diabetes care in Australian Indigenous communities. BMC Health Services Research 2007; 7: 67.
4. World Health Organization. Primary health care. Report of the international conference. Alma-Ata. Geneva: WHO, 1978.
5. Howanitz J, Howanitz P. Timeliness as a quality attribute and strategy. American Journal of Clinical Pathology 2001; 116: 311-315.
6. Kenagy JW, Berwick DM, Shore MF. Service quality in health care. Journal of the American Medical Association 1999; 281(7): 661-665.
7. Astion ML, Shojania KG, Hamill TR, Kim S, Ng VL. Classifying laboratory incident reports to identify problems that jeopardize patient safety. American Journal of Clinical Pathology 2003; 120: 18-26.
8. Shephard MDS, Gill J. The national QAAMS Program - a practical example of PoCT working in the community. Clinical Biochemist Reviews 2010; 31: 95-99.
9. Shephard MDS, Gill J. The analytical quality of point-of-care testing in the 'QAAMS' model for diabetes management in Australian Aboriginal medical services. Clinical Biochemist Reviews 2006; 27: 185-190.
10. Shephard M. Clinical and cultural effectiveness of the 'QAAMS' point-of-care testing model for diabetes management in Australian Aboriginal medical services. Clinical Biochemist Reviews 2006; 27: 161-170.
11. Shephard MDS, Gill J. Results of an innovative education, training and quality assurance program for point-of-care HbA1c testing using the Bayer DCA 2000 in Australian Aboriginal community controlled health services. Clinical Biochemist Reviews 2003; 24: 123-131.
12. Atkinson D, Murray R, Couzos S. Diabetes. In: S. Couzos, R. Murray (Eds). Aboriginal primary health care. South Melbourne, VIC: Oxford University Press, 2008; 521-574.
13. Shephard M, Spaeth B, Mazzachi B, Auld M, Schatz S, Loudon J, et al. Design, implementation and initial assessment of the Northern Territory Point-of-Care Testing Program. Australian Journal of Rural Health 2012; 20: 16-21.
14. Shephard M, Spaeth B, Auld M, Schatz M, Lingwood A, Loudon J, et al. Towards sustainable point-of-care testing in remote Australia - the Northern Territory i-STAT Point-of-Care Testing Program. Point-of-Care: The Journal of Near-Patient Testing and Technology 2014; 13(1): 6-11.
15. Shephard M, Mazzachi B, Shephard A, Burgoyne T, Dufek A, Ahkit J, et al. Point-of-care testing in Aboriginal hands - a model for chronic disease prevention and management in Indigenous Australia. Point-of-Care: The Journal of Near-Patient Testing and Technology 2006; 5: 168-176.

