Introduction
Although dental caries affects most people, its prevalence greatly differs throughout the world1. These differences are attributed to differences in risk factors, such as diet or the availability of fluoridated water2. Indeed, an inverse relation has been observed between the concentration of fluoride in drinking water and the presence of caries3. In addition to traditional biological factors, social and cultural factors have been included in the analysis of the risk factors for caries and periodontal disease4. One of these factors is living in a rural area, which leads to inequities in education and health, among others, and limits access to services5. In addition, rural areas are associated with low income6, which is also a major risk factor for these diseases7. Social factors, such as a large rural population and limited access to health services, might be associated with major risk factors for caries in children and teenagers8,9, but the evidence is limited.
Adolescence is a crucial stage in human development, during which oral health plays an important role. This vital stage is characterized by maturity and growth, both physical and psychological. From a dental perspective, teenagers are recognized as unique subjects10. In fact, the WHO has proposed age groupings for the assessment of oral health11 of 6, 12, 15, 35–44 and 65–74 years, two of them within the period of adolescence. Adolescents experience an increased risk for caries, traumatisms and periodontal diseases compared to younger children. Furthermore, this crucial period is commonly accompanied by poor nutritional habits, which contribute to the unique social and psychological needs of this population12. Based on the latter, it is necessary to develop preventive and therapeutic strategies specifically targeted for this population.
Dental caries affects 60–90% of school-aged children and nearly 100% of adults worldwide13. In addition, untreated dental caries is the most prevalent non-communicable disease in humans, affecting approximately 35% of the world’s population14. Periodontal diseases also have a high prevalence and can affect up to 90% of the world’s population15. While there is a perception that the incidence of caries may have decreased in some age groups, it remains high during adolescence16. For example, decayed, missing, filled teeth (DMFT) in 12-year-old children in Latin America is between 2.7 and 4.4, which is considered moderate13. For periodontal diseases, a significant percentage of the world’s infant and teenage population presents signs of gingivitis13. In Chile, a national DMFT of 1.9 (standard deviation (SD) 2.2) and a 77% prevalence of gingivitis at the age of 12 years were reported in 2007. These numbers correspond to a national study with a largely urban population and it is representative only of the entire country, not of individual regions. Given the significant role of rurality as a risk factor for disease17, the oral health of teenagers needs to be carefully monitored, considering specific factors, such as rurality. Because of the lack of available information from large regions of the country on the oral health of this population, the aim of this study was to determine the oral health status in 12- and 15-year olds in the Maule region in central Chile, one of the most rural of Chile, identifying potential risk factors for oral disease due to rurality.
Methods
A cross-sectional study was carried out in the Maule region of central Chile. It is important to note that the results reported in the present article are part of a larger International Association for Dental Research project: Regional Development Program for Training in Research Methods and Oral Health Surveys and The Assessment of Oral Health in the Chilean Division of the International Association for Dental Research, the EpiMaule study. The aim of the EpiMaule project was to assess the oral health status and treatment needs of the inhabitants of this area of Chile. The main study considered a representative sample of the approximately one million people living in the Maule region, using the age criteria set by the WHO: 6, 12, 15, 35–44 and 65–74 years11. This study reports only the information gathered from 12- and 15-year-old children, who the authors consider representative of the adolescents living in the region.
The sample size was calculated using regional data. To estimate the population in the Maule region, projection data for 201018 were obtained from the last official census in 2002. A total of 32.746 12- and 15-year-old children were projected to live in the region by 2011. The sampling process was stratified by age to estimate proportions and took into consideration caries prevalence in Chile19. The presence or absence of fluoride in the drinking water was used as a proxy to define rurality20, with a 95% confidence level and 3% estimation error. Using cluster analysis, the 30 districts of the region were divided into four groups according to rurality and population size. A population-proportional number of the 19 districts were allocated to each cluster. Schools were randomly selected from the official register of the Ministry of Education. In each school, adolescents were randomly selected from the class lists using a table of random numbers. Adolescents who behaved poorly during the examination, were sick on the day of their exam, or had systemic diseases that prevented them from having a periodontal examination, were excluded from the study. The calculated sample sizes were 552 and 486 adolescents of 12 and 15 years, respectively. Data collection extended from March 2011 to October 2011.
Examinations
A survey was used to collect information regarding age, sex, residential area, type of school and medical history. To determine oral status, each adolescent was examined by one of four calibrated examiners using the WHO criteria for oral health studies11. Exams were carried out without radiographs using a community periodontal index (CPI; WHO) probe, mirrors and LED front lamps and followed standard infection-control procedures. To access rural locations, schools were invited to participate by the coordinator of the study, who contacted the responsible person at each school. To avoid bias, rural and urban adolescents were examined using an identical protocol, independently of the existing physical conditions at the school. The DMFT index was used to assess caries status. The gingival exam included the oral hygiene index (OHI) of Silness and Löe and the gingival index (GI) of Löe and Silness11. The clinical attachment loss (CAL) and the CPI were also used in the examination for the 15-year-old group only, to prevent periodontal pockets from being confused with the deep gingival sulcus associated with eruption. A CAL score of 0 means an attachment loss of no more than 3 mm. A score of 1 indicated that participants had an attachment loss of between 4 and 5 mm, whereas a score of 2 indicated a loss of between 6 and 8 mm, and 3 meant an attachment loss of 9–11 mm, while 4 indicated an attachment loss of 12 mm or more. Hard copies of all records and information on the participants were kept by the principal investigator (RAG).
Calibration by examiners
Examiners were calibrated by two expert clinicians for DMFT and GI. The examiners’ calibration was conducted in DMFT in 12-year-old children (kappa inter- and intra-examiner agreement of 0.9 in both cases). In addition, a calibration was performed for the periodontal indexes in adult subjects (kappa inter- and intra-examiner agreement of 0.87 for both the OHI and the GI).
Statistical analysis
Fisher’s exact test was used to analyse the information. Statistical comparisons of medians and means were performed with the Mann–Whitney U-test to evaluate the significance. To determine the association between the caries variable and the variables of the OHI and GI, Spearman’s correlation was used. The information was processed and manipulated using the Statistical Package for the Social Sciences v14.0 for Windows (SPSS; http://www.spss.com).
Ethics approval
The study protocol was approved by the Bioethics Committee of the University of Talca (no. 00038). An informed consent form designed for this study was given to the legal guardian of each participant. Only those subjects who provided signed informed consent were included in the study. The final sample comprised 552 12-year-olds and 486 15-year-olds.
Results
Sample population was 51.1% women and 48.9% men among the 552 12-year-olds. Of those subjects, 42.6% lived in urban communities, and 57.43% lived in rural communities. In the group of 486 15-year-olds, 51.8% were women and 48.2% men, with 42.8% urban and 57.2% rural. Caries prevalence for the 12-year-olds was 61.77% (Table 1). Twelve year-old children from urban areas had prevalence of 54.04%, which was significantly lower (p<0.05) than the prevalence of 67.5% in rural areas. The decayed, missing, filled surfaces (DMFS), DMFT and SiC indexes at the age of 12 years were 4.43 (SD 4.39), 2.91 (SD 2.55) and 5.62 (SD 2.55), respectively (Table 1). Participants from rural areas had a significantly higher DMFT (5.25; SD 4.78) than their urban counterparts (3.32; SD 3.51) (p=0.0001). Similarly, the DMFT of the 12-year-old rural children was significantly higher (3.36; SD 2.71) than that of urban children (2.29; SD 2.17) (p=0.0001). The SiC in the rural sector was 6.21 (SD 2.44) whereas urban children showed 4.71 (SD 1.74) (p=0.0001).
For the 15-year-old adolescents, caries prevalence was 68.51% (Table 1). Children in urban communities had a prevalence of 64.59%, which was significantly lower (p<0.05) than the 73.58% in adolescents from rural communities. The DMFS, DMFT and SiC indexes at the age of 15 years were 7.87 (SD 8.29), 4.81 (SD 3.59) and 8.8 (SD 2.71), respectively (Table 1). Rural teenagers had a DMFS of 8.24 (SD 9.29) and the urban children of 7.58 (SD 6.78) (p=0.179). The DMFT of the 15-year-old rural children was 5.03 (SD 3.61) and that of the urban children was 4.65 (SD 3.58) (p=0.238). Caries prevalence among those more affected by the disease in both rural and urban areas was very high and not statistically different (p=0.128). While SiC index in the rural subjects of this age group was 9.16 (SD 2.26), urban adolescents had an index of 8.51 (SD 3.00).
Table 1: Caries prevalence, DMFT, DMFS and SiC of 12- and 15-year-olds in the Maule region, Chile, distributed for rurality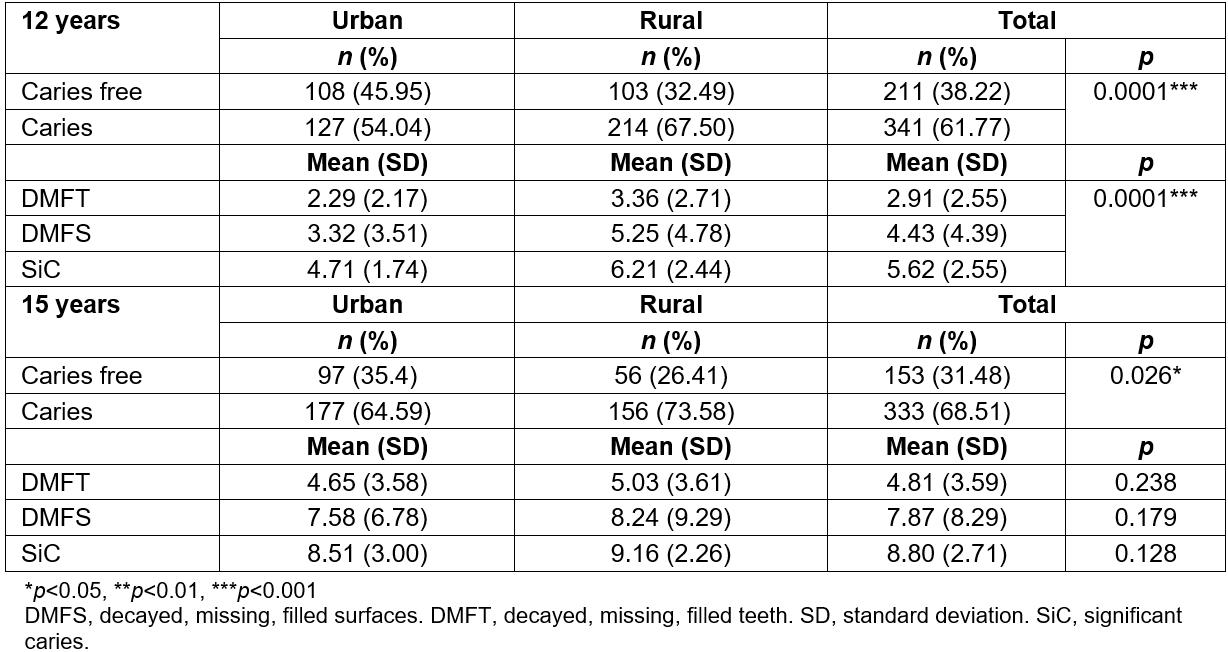
Periodontal indexes
The average of the OHI observed for the 12-year-olds was 1.47 (SD 0.41). The 12-year-olds from rural communities had an average OHI of 1.53 (SD 0.40), which was statistically significantly higher (p=0.001) than that of the urban group (1.4; SD 0.41) (Table 2). The average value of the GI of the 12-year-old group corresponded to moderate inflammation, with an average of 1.51 (SD 0.33). Significant differences were observed (p=0.002) between the rural teenagers (1.55; SD 0.34) and their urban counterparts (1.45; SD 0.29) (Table 2).
For 15-year-olds, the OHI average was 1.26 (SD 0.45). No significant differences in the OHI were observed between urban and rural 15-year-olds (p=0.418). While urban teenagers had an OHI of 1.27 (SD 0.44), rural individuals showed an OHI of 1.25 (SD 0.45) (Table 2). The average of the GI in the 15-year-old group indicated moderate inflammation, with an average of 1.43 (SD 0.34). No significant differences (p=0.624) were detected when urban and rural adolescents were compared. Subjects in rural communities had a GI of 1.44 (SD 0.33) compared to 1.42 (SD 0.35) in the urban group (Table 2).
CAL index was only assessed in 15-year-old adolescents. Most of these subjects (70.2%) had a CAL of 0–3 mm, followed by 28.8% with a CAL of 4–5 mm (Table 3). Statistically significant differences were not observed between the two groups (p=0.93).
According to the results of the CPI for the 15-year-old group, the presence of calculus or other retentive factors was most frequently observed. In the analysis, no statistically significant differences were observed between the urban and rural groups (p=0.06). Rural adolescents showed slightly higher indicators, with 2.13% more subjects had periodontal pockets of 4–5 mm than those from urban areas (Table 3).
Table 2: Gingival/oral hygiene indexes in 12- and 15-year-olds in the Maule region, Chile, according to place of residence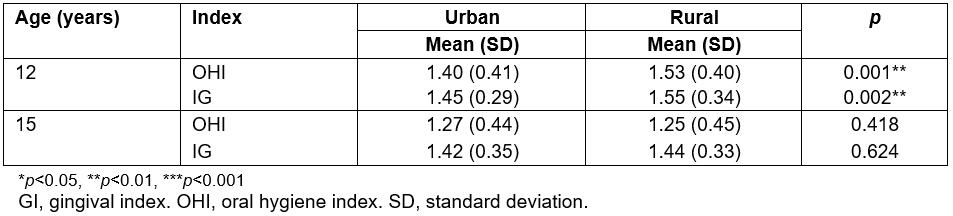
Table 3: Indicators of periodontal state in 15-year-olds in the Maule region, Chile, according to place of residence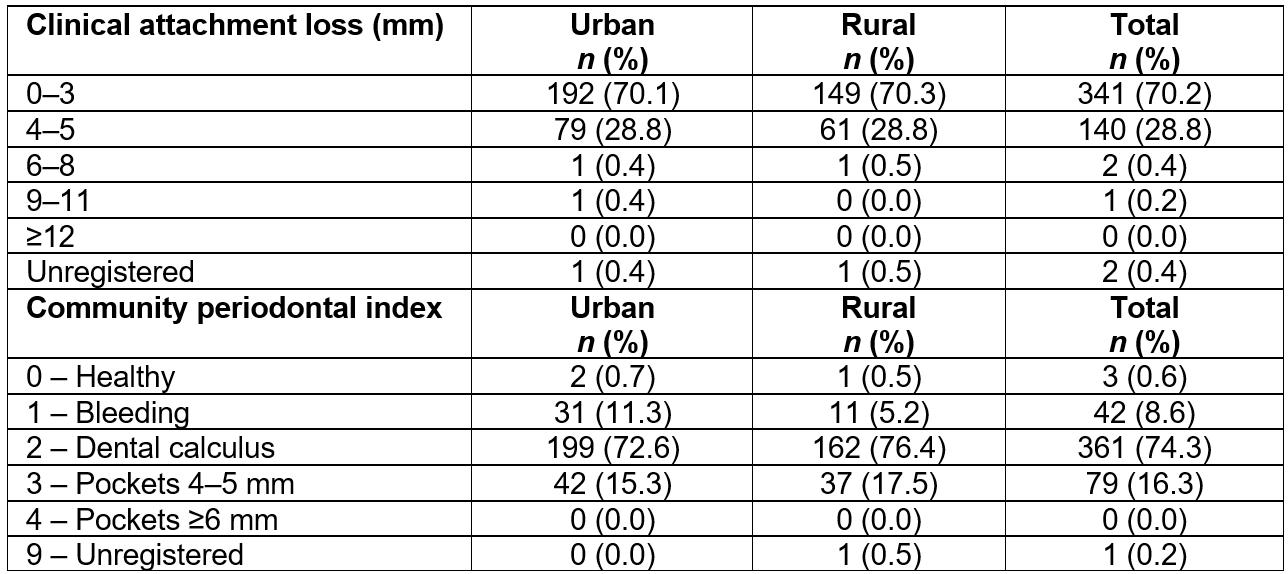
Correlation between caries, index of oral hygiene and gingival index
Due to the discrepancy observed between the urban and rural populations for caries and, less significantly, for the indicators of periodontal health, data were analysed to determine whether an association exists between caries, OHI and GI variables. In both the 12- and 15-year-old groups, a positive and significant correlation between caries and the OHI and GI was found (p<0.05), which would indicate that these quantitative variables are weakly associated with one another (correlation coefficients 0.183 and 0.084 for OHI and GI in 12-year-olds and 0.192 and 0.185 for OHI and GI, respectively, in 15-year-olds; Table 4). Therefore, if these two indexes increased, the prevalence of caries would also increase.
Table 4: Correlation between caries, oral hygiene index and gingival index in 12- and 15-year-olds in the Maule region, Chile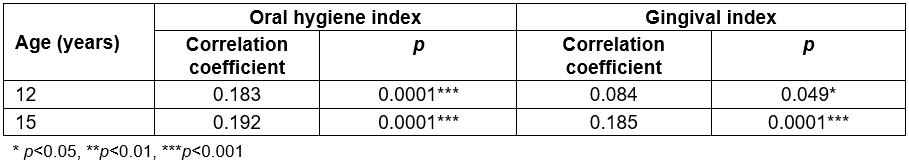
Discussion
This is one of the few studies in Chile with regional representation on the oral health of rural and urban teenagers. Twelve years is an index age used by WHO to compare oral health among countries and specific regions. In that context, these epidemiological data are a contribution to the characterization of a particular population in Chile with a strong rural component. Furthermore, a previous report on Chile’s oral health from the Health Ministry, but without regional representation, had indicated that the Maule region had a high caries prevalence21.
The EpiMaule study found that in 12-year-old children in the Maule region, caries prevalence of 62.5%, slightly lower than that reported in the latest national study22 of 63%. When compared to information from other countries, the Maule region showed lower prevalence than Lima, Peru with 83.3%23, but is higher than Saudi Arabia24, Germany25 and China26. As for the 12-year olds, 15-year-old adolescents from the Maule region had caries prevalence of 68.5%, which is lower than that reported in another regional study in Chile with 73.9%, which showed a prevalence of caries of 73.9%27. Compared with international studies, caries prevalence was higher than in Salvador of Bahia, Brazil, with 65.1%28 and India, with 46.7%29. Caries prevalence in the Maule region was lower, however, than that reported for North-East Russia, with 91.8%30.
In terms of the DMFT, the EpiMaule study showed higher scores than those reported for 12-year-olds in Brazil31 and in Chile 32. From data of the EpiMaule study33, a sudden increase in caries experience may be observed between ages 6 and 12 years, as indicated by a DMFT of 0.24 (SD 0.74) at the age of 6 years and of 2.91 (SD 2.55) at the age of 12 years. High caries prevalence and history in adolescents indicates an important oral damage, which, combined with rurality, low socioeconomic level, low access to care and limited options for restorative treatment, strongly suggests the need for more effective preventive measures focused on children and adolescents and that consider rurality.
The DMFT of the 15-year-old group was higher than the national average of 3. No information was available in the country for 15-year-old rural adolescents. This information is relevant, as it portrays oral health of a community that may be at a different risk than the urban counterparts. Indeed, higher caries prevalence has been reported in rural areas in Poland34, Georgia35 and Nigeria36. These global inequalities in oral health highlight the importance of some social and structural determinants and the conditions of daily life in the health of certain populations, including economic differences between and within countries37.
Results for the CPI indicated that most of the teenagers had calculus or other retentive factors, but not periodontal pockets. This is consistent with information reported in other countries38,39. For the CAL index, the 0–3 mm range had the highest prevalence (70.16%), which might be explained by the absence of appropriate oral hygiene. In this age group, teeth have been in the mouth for only a few years, which may explain the lack of more severe damage in the periodontal tissues. No other studies have used these indexes in 15-year-olds at either the regional or national level. This information might be useful as a reference for future investigations and as a regional or national baseline for the implementation of oral health policies.
In terms of the periodontal indexes, the OHI in the 12-year-old group and the GI in both groups found poorer periodontal conditions in the rural than in the urban population. Rural populations typically have more limited access to dental care and to preventive tools, such as toothbrushes and dentifrices5, mainly due to economic constraints. Differences between the rural and urban populations can be attributed to the different contexts in which these subpopulations develop in terms of determinants of social health40. These include the political and socioeconomic context that contributes to differences in functionality and culture, as well as socioeconomic factors, such as income, which has been shown to be inversely associated with a major risk of oral disease8. On an individual level, besides the biological factors typically considered to assess oral health, it is important to include the social environment and psychosocial factors. Thus, oral health may be the result of the behaviour of an individual, including habits and diet, and the material circumstances, such as the type of housing and access to services like drinkable water40.
It has been pointed out that rurality in Chile might be a risk factor for detrimental oral health, because individuals who live in rural sectors present poorer indicators of oral health than those in urban sectors22,41. Differences in oral health indicators in rural individuals, such as those shown here, may derive from limited access to essential oral health care, economic and geographical barriers, as well as to low levels of education, less healthy ways of life and lack of drinkable fluoridated water42. This study defined rurality based on access to fluoridated drinking water. Since urban children studied in the EpiMaule study have been exposed to fluoride in drinking water all their lives, differences between the rural and urban populations, particularly at the ages of 12 and 15 years, may be explained. Besides fluoride as a differentiating factor, the intrinsic characteristics of the rural population, such as lower education and socioeconomic levels, might strongly influence a higher prevalence of oral conditions43. Despite intense debate over the effectiveness of fluoridated water, this measure remains as the most effective and socially equitable measure for preventing caries from a public health standpoint. In the era of increasing access to other forms of fluoride, such as fluoridated dentifrices and professionally applied fluoride44, the efficiency of fluoridated water in the prevention of caries needs to be revisited45. However, the present study showed a difference between urban and rural communities of 13.5% in caries prevalence at the age of 12 years and 9% at the age of 15 years, between subjects. Considering that sampling in the EpiMaule study considered fluoride in the drinking water as the factor to differentiate urban from rural communities, this difference would be in approximately the same range of magnitude. The need to maintain fluoridation of drinking water is still under revision and needs further research before drawing definitive conclusions.
Studies that have attempted to associate GI with caries are controversial, and several researchers have found no association. Conversely, other reports have found a strong and significant correlation between these factors and others, such as socioeconomic status or the consumption of sugar46. This inconsistency might be attributed to the different methods used to measure oral hygiene or to the difficulty in determining whether the effect of brushing in caries control is due to the mechanical removal of the oral biofilm or from the addition of varying amounts of fluoride in the toothpaste47. The present study’s results failed to show a strong association between the periodontal indexes and the prevalence of caries.
Caries is a sugar and biofilm-dependent disease. Because the dental biofilm is ubiquitous, frequent sugar exposure is the aetiological factor48. Therefore, caries occurrence in teenagers can be attributed to high sugar consumption, which is a necessary factor for the initiation of caries, but may not be related to the indexes of periodontal health, as shown here. The differences between the urban and rural populations, however, may be attributable to a different fluoride exposure. Diverse risk factors for caries have been stated, such as socioeconomic status, sugar consumption, early acquisition of cariogenic microorganisms, and biofilm control. The present study measured biofilm control by means of the OHI and the GI. No other study had measured this correlation in teenagers at both 12 and 15 years of at either the regional or the national level. This information is key to differentiate the aetiology of dental caries and periodontal disease. Oral health preventive measures usually focus on improving toothbrushing, which may be appropriate to prevent periodontal disease. Also, these preventive programs or interventions usually invest important resources and efforts in providing fluoridated toothpaste. The most important aetiological factor in caries, sugar consumption, is usually ignored, nevertheless. The latter is of particular importance during adolescence. Even if the parents or caregivers limited sugar consumption during early childhood, teenagers develop levels of autonomy in their decisions and gain access to sugared food at school and from friends. Education, therefore, turns into a key component of any preventive program to raise awareness among adolescents of the risks implied in sugar consumption.
Besides serving as a baseline for prospective studies, the information obtained from this study should serve to support public policies tailored to adolescents as a separate group that needs individual preventive approaches. Moreover, oral health interventions should consider rurality, and all its associated issues as a particular situation that requires specific oral health measures. Increased access to care, by means of more health centres strategically located to reach local communities and the increase in the rate of dentists per number of inhabitants, is one of the main actions. Other key efforts policymakers and the states must take is to improve education, in general and specifically on health, among rural communities, particularly in the children and adolescent age groups. Prioritized actions targeted and tailored to rural communities will lead to reduced disparities.
Conclusions
Adolescents of the Maule region presents a situation of deficient and inequitable oral health. Rural teenagers without access to fluoridated water show poorer indicators for prevalence and caries experience, without noticeable differences in gingival health. Besides fluoride in the drinking water, social determinants associated with rurality may explain these differences. Policymakers should consider rurality in public health interventions, particularly during the critical period of adolescence. Policy focus should be increased access to care and better general and health education as the main target to reduce disparities in oral health.


