Clinical pathways (CPs) facilitate management of healthcare quality through standardization of care protocols. Their implementation decreases variability in clinical practice and improves therapeutic outcomes. They also promote organized and efficient patient care, which is founded on evidence-based practice1,2. Recent studies show that CPs optimize outcomes in pain management during palliative care, a major issue for cancer patients1,2. However, most studies focus on CPs in hospital-based palliative care; studies describing the use of CPs in home palliative care, especially in rural and remote areas, are scarce.
A previous study in Japan showed that approximately 80% of people wished to receive palliative care and die at home if they were affected by cancer; however, 60% believed that this would be impossible because of socioeconomic and medical barriers3. Recent studies have shown that cancer pain is one of the most severe barriers to realizing home palliative care4. World Health Organization pain management guidelines are recognized as effective in managing cancer pain, and opioids are positioned as the central drugs5. The Japanese Ministry of Health, Labour and Welfare and the Japanese Society for Palliative Medicine have begun to introduce lectures and clinical projects on cancer pain management; however, there is room to improve cancer pain management in Japan6. For example, the extent of opioid usage in Japan compared with other developed countries is extremely small7.
Kozu Island is a remote island in south-eastern Japan, located approximately 178 km from Tokyo. In performing home palliative care, physicians on Kozu Island frequently face difficulties characteristic to medical institutions in remote locations, such as lack of specialists in palliative medicine, poor knowledge of palliative care among medical staff, and inconsistency of care and care policy due to frequent changes of physicians. Previous reports describing palliative care in rural and remote areas highlight similar obstacles6-8.
Implementation of a CP for pain management in home palliative care in remote locations is reportedly an effective solution to the problems of maintaining quality of care and consistency of policy, while educating medical staff9-11. In this study, a CP for pain management in home palliative care settings on a remote island was implemented and its effectiveness examined.
Kozu Island and the Kozu National Health Insurance Clinic
The population of Kozu Island was 1985 in 2010, and 27 people died in that year. From 2006 to 2011, 44 people (26% of all decedents) died of cancer on Kozu Island, 24 (54%) of whom died at home12.
The Kozu National Health Insurance Clinic is the only medical institution on Kozu Island, employing two physicians and seven nurses. Physicians are engaged in outpatient care in the morning and house calls or medical examinations in the afternoon.
Almost all physicians in the clinic are graduates of Jichi Medical University (JMU) and are dispatched on a short-term (within 1 year) basis by the Tokyo Metropolitan Government because it is difficult for clinics in remote locations to secure physicians on a long-term basis. The assignment system of JMU physicians is reported elsewhere13.
The clinic has no beds so patients must go to other hospitals, for instance in Tokyo, for long-term hospitalization. Therefore, physicians provide home-based rather than hospital-based palliative care for terminal cancer patients.
Physicians introduced a 24 hour accessibility system to facilitate consistent home palliative care. At least one nurse is on duty at night and during holidays. Patients and family members can call anytime with concerns or requests. Duty nurses provide assistance or consult physicians if they cannot assist or if a medical assessment is required. Physicians then determine the best course of action.
As some terminal cancer patients cannot travel to the clinic because of pain, emaciation or cachexia, a home visit system was also introduced in which physicians visit such patients and treat them at home instead of at the clinic.
Subjects
This study reviewed 24 patients who received home palliative care from physicians and nurses of the Kozu National Health Insurance Clinic and who died of cancer during the period of April 2006 to December 2011 (Table 1). Performance status is the score of cancer patients' activities of daily life, and WHO performance status scores are used in this article14. The patients fell into two groups: pre-CP and post-CP (Fig1). The pre-CP group comprised 17 patients who died before the introduction of the pain management CP in April 2011; the post-CP group comprised seven patients who died after the CP implementation. Age, gender, and performance status were not significantly different between groups. Eleven patients (65%) in the pre-CP group and six (86%) in the post-CP group were prescribed opioids for cancer-related pain. One patient in the post-CP group was not prescribed opioids because cancer-related pain was absent.
Table 1: Patient characteristics at baseline
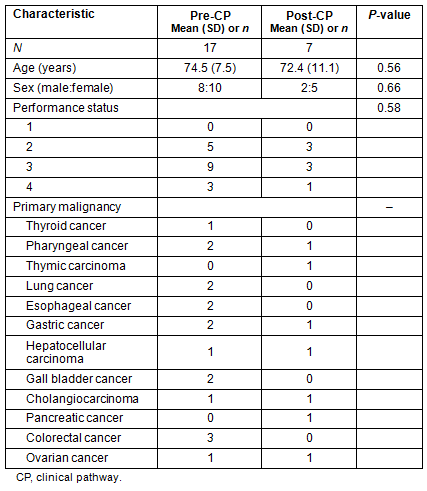
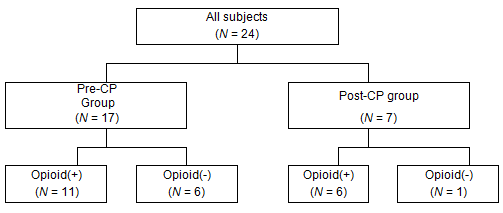
Figure 1: Group division of subjects. CP, clinical pathway.
The clinical pathway
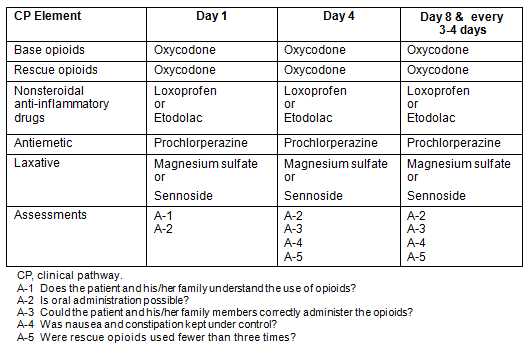
A pain management CP for home palliative care was introduced by the authors in April 2011 (Table 2) based on WHO pain management guidelines5. Lectures for other physicians and nurses were given on the significance of the CP before its introduction. The CP focused particularly on establishing a rescue dose of opioid, and combining nonsteroidal anti-inflammatory drugs (NSAIDs), antiemetics, and laxatives with opioids. The opioid prescribed was oral oxycodone. Base opioid was defined as the total daily prescription. Rescue opioid was defined as a dose of opioids for sudden, severe pain, prescribed at one-sixth of the dose of base opioids15. The NSAIDs prescribed were loxoprofen or etodolac; the antiemetic was prochlorperazine; and the laxative was magnesium sulfate or sennoside. Assessment at day 1 comprised the following questions:
- Does the patient and his/her family understand the use of opioids?
- Is oral administration possible?
While on day 4, 8 and every 3-4 days thereafter, the assessment comprised:
- Is oral administration possible?
- Could the patient and his/her family correctly administer the opioids?
- Was nausea and constipation kept under control?
- Were rescue opioids used fewer than three times?
Assessment of side-effects or drug compliance mentioned was performed during a medical interview conducted by physicians, or during a telephone consultation with physicians or nurses. If patients or their family members called on an unscheduled day in an emergency, assessment was performed according to the CP.
If the assessment was not fulfilled, the following actions were taken:
- opioid use was explained again
- alternative methods of opioid administration - for example, patches or injections - were considered
- alternative drugs of antiemetics and laxatives were considered
- doses of the base and rescue opioids were increased.
To educate staff effectively, the actions of the physicians and nurses were evaluated after each assessment; if found that they had not followed the CP, they were then re-educated. Physicians' actions were assessed by a nurse and nurses' actions were assessed by a physician.
Table 2: Clinical pain management pathway
Outcomes and endpoints
Clinical pathway effectiveness and pain management quality were evaluated. Two medical staff from Kozu National Health Insurance Clinic other than the authors retrospectively reviewed the medical records of the present patients in January 2012. To evaluate CP effectiveness, patients in both groups who were set a rescue dose and who were also prescribed NSAIDs, antiemetics and laxatives in combination with opioids were compared. This evaluation was performed only for patients who were prescribed opioids because physicians did not need to prescribe antiemetics or laxatives for those not prescribed opioids, Thus, the evaluation was restricted to eleven patients in the pre-CP group and six in the post-CP group. To evaluate pain management quality, authors compared Pain Management Index (PMI) scores at: day 1 (baseline); day 8 following CP initiation; and within 3 days of death16. The evaluations on day 8 and within 3 days of death represented assessments of primary pain treatment and pain control just before death, respectively. One of the significant goals of home palliative care is a peaceful death at home, and the quality of pain control just before death is a very important factor. Pain Management Index score calculation is explained (Table 3). Scores of 0, 1, 2, and 3 were assigned when patients reported no (0), mild (1-3), moderate (4-7) or severe pain (8-10), respectively, based on the Edmonton Symptom Assessment System or Brief Pain Inventory17. Analgesic scores of 0, 1, 2 and 3 were assigned for no pain medication, nonopioids, weak opioids and strong opioids, respectively. Negative PMI suggests inadequate pain management16.
Table 3: Pain Management Index
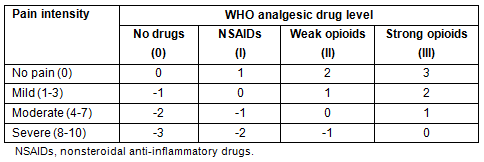
Statistical analysis
Fisher's exact test was used to compare the ratio of patients who were set a rescue dose, and NSAIDs, antiemetics and laxatives between the groups. We used the Mann-Whitney U-test to compare PMI scores between groups. All statistical analyses were performed using Microsoft Excel 2010. All statistical tests used were two-sided. A p-value of <0.05 was considered statistically significant.
Ethics approval
The present study was approved by the institutional review board of Kozu Island Clinic and Community Health and Welfare division of Kozu village (No. 2011-02). Informed consent for patients was provided by physicians before implementing the CP.
After initiation, no patient deviated from the CP. The proportion of patients in whom a rescue dose was set was 100% in the post-CP group versus 46% in the pre-CP group (Table 4). The proportion was significantly higher in the post-CP group (p=0.04). The use of NSAIDs with opioids and the use of antiemetics and laxatives with opioids were 100% and 100% in the post-CP group versus 18% and 27% in the pre-CP group, respectively (Table 4). The proportion was significantly higher in the post-CP group (p=0.002 and p=0.009, respectively).
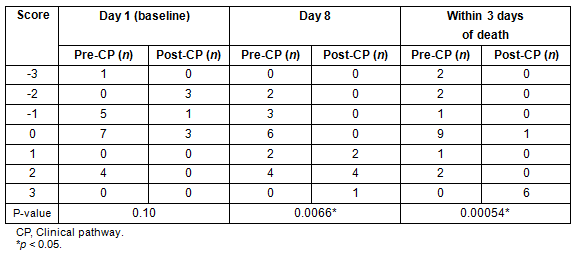
Table 5 shows the distribution of PMI scores at day 1 (baseline), day 8 following CP initiation, and within 3 days of death. Baseline PMI scores were not significantly different between groups (p=0.1); however, scores at day 8 and 3 days before death were significantly higher in the post-CP group (p=0.007 and p=0.0005, respectively). The proportion of negative PMI scores at day 8, indicating inadequate pain management, was 0% in the post-CP group versus 29% in the pre-CP group (p=0.27), and that within 3 days before death was 0% in the post-CP group versus 29% in the pre-CP group (p=0.27).
Table 4: Rescue dose and combination of nonsteroidal anti-inflammatory drugs,
antiemetics and laxatives with oxycodone, according to clinical pathway
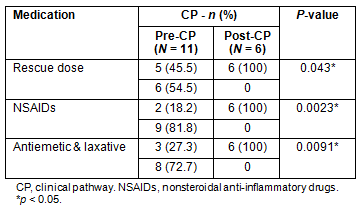
Table 5: Pain Management Index at day 1 (baseline), day 8, and within 3 days before death
Discussion
Based on the present results, implementation of a pain management CP in home palliative care settings in remote locations improves compliance with the WHO pain management guidelines5 and pain management quality of terminal cancer patients. Most subjects of recent studies about CPs in palliative care are patients in hospitals or hospices18, and few studies have evaluated the effectiveness of CPs for home palliative care in remote areas. The present study shows that CPs are equally effective in home palliative care settings in remote areas.
The WHO pain management guidelines are based on clinical evidence5. Many studies declare WHO guidelines to be effective in the management of cancer-related pain, and similar guidelines have been drafted based on WHO pain treatment policies19,20. Thus, physicians engaged in palliative care are required to be familiar with WHO pain management guidelines; however, a number of physicians lack knowledge of the guidelines and perform inadequate pain treatment for cancer patients21,22. This is more prevalent in rural and remote areas23,24. The present study shows that compliance with WHO pain management guidelines and pain management quality were poor before CP implementation on a remote island in Japan. Two difficulties that are particular to remote locations account for this inadequate management.
First, it is challenging for clinics in remote locations to attract physicians to practice in these areas in the long-term, and much more difficult to secure palliative care specialists. Although the number of palliative care specialists in Japan recently increased, it is still insufficient to meet the demands of both urban and rural areas25. Rural and remote areas often face a shortage of physicians26. The Kozu National Health Insurance Clinic uses a short-term rotation system to cope with a shortage of physicians. A rotation system is a good strategy for remote areas; however, it results in inconsistency in pain management. Additionally, palliative medicine specialists have never been dispatched to remote locations, even when a rotation system is implemented.
Second, the provision of education for nurses, who play a key role in the coordination and delivery of community-based palliative care in rural and remote areas and in our 24 hour accessibility system, is inadequate. Because the quality of home palliative care on Kozu Island depends not only on physicians but also on nurses, their education is very important. Palliative care education for nurses is improving27 but only in urban areas28,29 and it is still far from satisfactory in remote locations. As a result, many nurses may not be able to provide adequate assistance, in line with WHO pain treatment guidelines, to patients or their families.
The present study suggests that CP implementation can overcome such difficulties of remote location. Physicians and nurses are encouraged by the authors to use WHO pain management guidelines and follow the same therapeutic policy for home palliative care, resulting in decreased variability in treatment, consistency in clinical policy and standardized evidence-based treatment; moreover, CPs are also educational for medical staff9-11. By treating patients according to a CP, physicians and nurses gain experience in evidenced-based home palliative care and receive feedback on their actions through the assessment. Consequently, CPs effectively lead to perceptible improvements in palliative care quality.
On the basis of the findings, we propose two recommendations. First, physicians in locations where home palliative care is not sufficiently widespread should consider introducing CPs. Second, CPs should be used by physicians to teach evidence-based or guideline-based medicine to medical staff in rural and remote areas.
This study has several limitations. First, the observations were made for only 1 year following CP introduction; the efficiency of the CP may have been clearer if authors had continued observing for several years. Second, the CP itself should be evaluated, modified and updated regularly. Further study is required to determine appropriate CP modifications and updates. Third, some biases in evaluating CP effectiveness and pain management quality may have arisen because the medical staff at the clinic referred to medical records. Forth, the number of all subjects is small and further studies with larger numbers may be required to replicate our results.
This study showed that a pain management CP in home palliative care improved compliance with WHO pain management guidelines and quality of pain management. The CP is a useful tool to overcome difficulties that are particular to home palliative care in remote locations and teach evidence-based medicine to medical staff.
Acknowledgments
The authors thank Dr Yasuyuki Miyazaki (Kozu National Health Insurance Clinic) and the Clinical Research Support Team Jichi for their advice.
References
1. Yamada T, Furukawa K, Yokoi K, Mamada Y, Seya T, Makino K et al. Clinical pathway for oral controlled-released oxycodon in digestive cancer patients. Japanese Journal of Gastroenterological Surgery 2009; 42: 1448-1453.
2. Apolone G, Corli O, Caraceni A, Negri E, Deandrea S, Montanari M et al. Pattern and quality of care of cancer pain management. Results from the Cancer Pain Outcome Research Study Group. British Journal of Cancer 2009; 100: 1566-1574.
3. A survey on perception of lay people for hospice and palliative care in 2008. Japan Hospice Palliative Care Foundation (in Japanese). (Online) 2009. Available: http://www.hospat.org/research-202.html (Accessed 24 October 2011).
4. Kenji E. Development of palliative medicine for cancer patients in Japan: from isolated voluntary effort to integrated multidisciplinary network. Japanese Journal of Clinical Oncology 2010; 40(9): 870-875.
5. World Health Organization. WHO's pain ladder. Geneva, Switzerland: World Health Organization, (Online) 2011. Available: http://www.who.int/cancer/palliative/painladder/en (Accessed 24 December 2011).
6. Japanese Society of Palliative Medicine. About the peace project. Japanese Society for Palliative Medicine (in Japanese) (Online) 2011. Available: http://www.jspm-peace.jp/about.html (Accessed 4 January 2012).
7. Ministry of?Health, Labour and Welfare. Narcotics for Medical Use. Cancer Statistics in Japan 2010. Foundation For Promotion of Cancer Research (in Japanese) (Online) 2011. Available: http://www.fpcr.or.jp/pdf/statistics/date12.pdf (Accessed 4 January 2012).
8. Kelley ML, Williams A, DeMiglio L, Mettam H. Developing rural palliative care: validating a conceptual model. Rural and Remote Health 11: 1717. (Online) 2011. Available: www.rrh.org.au (Accessed 28 October 2012).
9. Duffy A, Payne S, Timmins F. The liverpool care pathway: does it improve quality of dying? British Journal of Nurses 2011; 20(15): 942-946.
10. Gordon DB. Critical pathways: a road to institutionalizing pain management. Journal of Pain Symptom Management 1996; 11: 252-259.
11. Chalkidou K, Lord J, Obeidat NA, Alabbadi IA, Stanley AG, Bader R et al. Piloting the development of a cost-effective evidence-informed clinical pathway: managing hypertension in Jordanian primary care. International Journal of Technology Assessment in Health Care 2011; 27(2): 151-158.
12. Inoue K, Hirayama Y, Igarashi M. Trends of diseases of residents: health promotion plan in Koze island 2011. Tokyo: Medical Education, 1997 [In Japanese].
13. Inoue K, Hirayama Y, Igarashi M. A medical school for rural areas. Medical Education 1997; 31(6): 430-434. Available: http://www.ncbi.nlm.nih.gov/pubmed/9463645 (Accessed 23 July 2012).
14. Oken MM, Creech RH, Tormey DC. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982; 5(6): 649-655.
15. Japanese Palliative Care Association. Method of palliative care. Japanese palliative care association. (Online) 2008. Available: http://gankanwa.jp/tools/step/data2/index.html#data003 (Accessed 24 December 2011).
16. Philip J, Smithi WB, Craft P, Lickiss N. Concurrent validity of the modified Edmonton Symptom Assessment System with Rotterdam Symptom Checklist and the Brief Pain Inventory. Supportive Care in Cancer 1998; 6(6): 539-541.
17. Eguchi K. Development of palliative medicine for cancer patients in Japan: from isolated voluntary effort to integrated multidisciplinary network. Japanese Journal of Clinical Oncology 2010; 40(9): 870-875.
18. Mitera G, Fairchild A, DeAngelis C, Emmenegger U, Zurawel-Balaura L, Zhang L, et al. A multicenter assessment of the adequacy of cancer pain treatment using the pain management index. Journal of Palliative Medicine 2010; 13(5): 589-593.
19. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncologic pain. Fort Washington, PA: National Comprehensive Cancer Network. (Online) 2001. Available: http://www.nccn.org/international/international_adaptations.asp (Accessed 24 October 2011).
20. Jost L, Roila F, ESMO Guidelines Working Group. Management of cancer pain: ESMO clinical recommendations. Annals of Oncology 2009; 20: 170-173.
21. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists' attitudes and practice in cancer pain management: a national survey. Journal of Clinical Oncology 2011; 29(36): 4769-4775.
22. Bernabei R, Gambassi G, Lapane K, Landi F, Gatsonis C, Dunlop R et al. Management of pain in elderly patients with cancer. JAMA 1998; 279(23): 1877-1882.
23. Joseph N, Jayarama S, Kotian S. A comparative study to assess the awareness of palliative care between urban and rural areas of Ernakulum district, Kerala, India. Indian Journal of Palliative Care 2009; 15(2): 122-126.
24. Kaufman J, Forman WB. Hospice and palliative care: an educational intervention for healthcare professionals in a rural community. American Journal of Hospice and Palliative Care 2005; 22(6): 415-418.
25. Akiyama M, Matoba M, Takebayashi R, Nakame C, Matubara Y. The research of home palliative care in community. Palliative Care Research 2009; 4(2): 112-122.
26. Tanihara S, Kobayashi Y, Une H, Kawachi I. Urbanization and physician maldistribution: a longitudinal study in Japan. BMC Health Service Research 2011; 11: 260.
27. Ishikawa H, Kawagoe K, Kashiwagi M, Yano E. Nurse-physician collaboration in pain management for terminally ill cancer patients treated at home in Japan. Journal of Palliative Care 2007; 23(4): 255-261.
28. Kizawa Y, Tsuneto S, Tamba K, Takamiya Y, Morita T, Bito S et al. Development of a nationwide consensus syllabus of palliative medicine for undergraduate medical education in Japan: a modified Delphi method. Palliative Medicine 2011; 9: 15.
29. Carlson G. Clinical pathways in terminal care. Do clinical pathway for terminal care improve symptoms and quality of life of patients and family? Pfledge Zeitschrift 2011; 64(9): 552-553 [In German].

