full article:
Gestational diabetes mellitus (GDM) is the most common antenatal condition complicating pregnancy in Western Australia (WA), with 5.1% of all women giving birth diagnosed with GDM1. It is now recognised that GDM even in mild forms may have an adverse impact on pregnancy outcomes2,3, including fetal macrosomia, obstructed labour, increased caesarean section and operative delivery rate and neonatal hypoglycaemia. It has also been shown that treatment reduces adverse outcomes4. There are no published figures for rural or regional antenatal morbidity and in particular for GDM rates within WA. However, rates of rural antenatal morbidity might be expected to be higher than rates for urban areas for similar reasons, explaining the difference in perinatal mortality rates5. In particular, rates of GDM might be expected to be higher in rural areas due to increased rates of obesity6,7, higher proportion of Aboriginal people with an associated higher susceptibility to GDM8, and poorer access to health services for screening, monitoring and treatment of GDM in pregnancy. Quantifying the rate of GDM in rural areas is the first step in addressing the adverse effects of this important complication of pregnancy.
Identifying the most significant risk factors is also important in case finding and identifying women most likely to benefit from intervention. Well-recognised risk factors for GDM include obesity, increasing maternal age, ethnicity, previous fetal macrosomia and family history of gestational diabetes or type 2 diabetes. Socioeconomic status is a recognised risk factor for GDM9,10, as it is for type 2 diabetes11.
Geographic location is a primary determinant of many rural health problems, being associated with socioeconomic status, ethnic background and access to health care and other services. While residential address is only a proxy for the usual measures of socioeconomic status, such as education and income, it does reflect an aggregate effect of these determinants. It is an important factor to consider when discussing rural health outcomes.
Bunbury Regional Hospital (BRH) is a referral base for rural south-west WA. The majority of patients reside in Bunbury or the immediate suburbs; however, the catchment includes towns north to Yarloop, east to Collie and Boyup Brook and south to Walpole. The patient population is reflective of a rural regional centre.
A retrospective review of the Stork obstetric database at BRH for the 2 year period from February 2009 to March 2011 was conducted. The Stork database is an electronic summary of antenatal and delivery details for all women giving birth. This database has been used in a number of other hospitals within WA; however, BRH is the first hospital outside the urban area to use it. Midwives enter data on all pregnancies at around 26 weeks gestation when women are first seen for booking into the obstetric unit and again at discharge postpartum. It includes details of antenatal tests, smoking and alcohol use, depression scores, delivery details such as mode of delivery, analgesia, perineal status and neonatal outcomes.
The study looked at antenatal risk factors for GDM and maternal and neonatal outcomes. Data were collected on maternal age, parity, birth plurality, BMI at booking, ethnicity, suburb of residence, presence of GDM, smoking during pregnancy, hypertension, pre-eclampsia and Edinburgh Postnatal Depression Score (EPDS) at booking. Data were also collected on birth outcomes, including birth method, shoulder dystocia, total blood loss in millilitres, length of stay in days, birth gestational age, birth weight, fractured clavicle, Erbs palsy, cephalohaematoma, neonatal jaundice, phototherapy, neonatal hypoglycaemia and admission to neonatal special care or intensive care units. In addition, babies who were large for gestational age (LGA) were identified according to standard Australian birth charts12.
The Socio-Economic Index for Areas (SEIFA) has been developed by the Australian Bureau of Statistics (ABS) as a measure of the welfare of Australian communities13. The use of SEIFA scores allows an approximate measure of socioeconomic status based on residential address. The SEIFA score was derived from tables from the ABS14. SEIFA decile scores were used to rank patients. The decile scores were then grouped into quintiles to give meaningful sample sizes. The lowest SEIFA quintile 1 corresponds with the lowest socioeconomic status. Presence of GDM was determined by routine screening for GDM at 26-28 weeks with a glucose challenge test. Those with a high glucose challenge test score underwent the 75 g oral glucose tolerance test. GDM was diagnosed if the test showed fasting blood sugar level (BSL)≥5.5 mmol/L or 2 hour BSL≥8.0 mmol/L, in accordance with accepted Australian criteria at the time of the study15. BRH maternity unit followed up missing results to ensure complete screening of the obstetric population.
Statistical analysis
Statistical analysis included descriptive analysis using χ2 and Fisher's exact test for univariate associations. Individual cell χ2 values were determined where a significant proportion of the table χ2 was attributed to this cell. Continuous variables such as gestational age at birth and blood loss were assessed using the Kruskal-Wallis (KW) test, as they were skewed and kurtotic. Variables were included in the stepwise logistic regression analysis with an entry level of significance of 0.2 and retained with a value of 0.05. The logistic regression analysis was used to obtain a logistic coefficient for each variable, and the score became the sum of these coefficients16.
The evaluation of Pi= e(constant+logistic coefficients)/1+ e(constant+logistic coefficients) gives the prediction of the occurrence of GDM for any patient i16. Analyses were conducted using Statistical Analysis Software v9.3 (SAS Institute, www.sas.com).
Ethics approval
Ethics approval for this study was obtained from the WA Country Health Service Board Research Ethics Committee (2010:24).
Of 1645 women delivered at BRH in the study period, nine had pre-existing diabetes and were excluded from further analysis. A further 73 (4.46%) developed GDM in the current pregnancy. Demographic and antenatal parameters are shown in Table 1, together with their associations with GDM. Maternal age, BMI, SEIFA quintile 1 and Asian ethnicity were significant univariate predictors of GDM. Although there is often an association between smoking status and SEIFA, in our population women of all status groups smoked and this is not significantly associated within the group of women with GDM.
Table 1: Demographic and antenatal factors and gestational diabetes mellitus
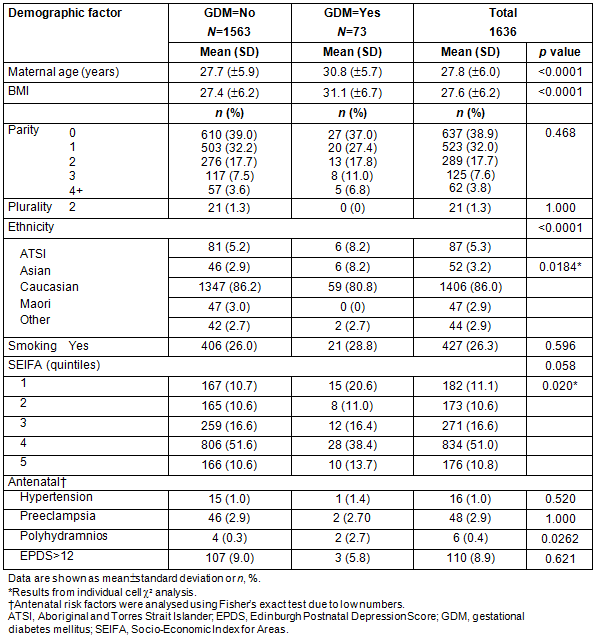
The results of logistic regression analysis are presented in Table 2. The dependent variable was presence of GDM in the pregnancy. All demographic or antenatal risk factors that showed an association with GDM at p<0.20 were initially included in the model, together with interactions of SEIFA with maternal age and BMI, and maternal age and BMI together. Ethnicity was modelled as Aboriginal and Torres Strait Islander (ATSI), Asian and Other, parity as 0-2 versus >3, and SEIFA as quintile 1 versus rest in line with the univariate results. Maternal age was modelled as <20 years and then in 5 year intervals, whereas BMI was modelled as underweight (<18.6), normal (18.6-25), overweight (25.1-30), obese (30.1-35) and very obese (>35.1). The first analysis showed there was no additional significance attached to the SEIFA interactions with maternal age (p=0.155), BMI (p=0.694), ethnicity (0.969) or parity (0.302) or for maternal age with BMI (0.611) over that already present for the variables alone, and the second analysis removed any parity effect (p=0.924). The final model is summarised in Table 2.
Table 2: Summary of logistic regression model with odds ratio estimates and profile likelihood confidence intervals
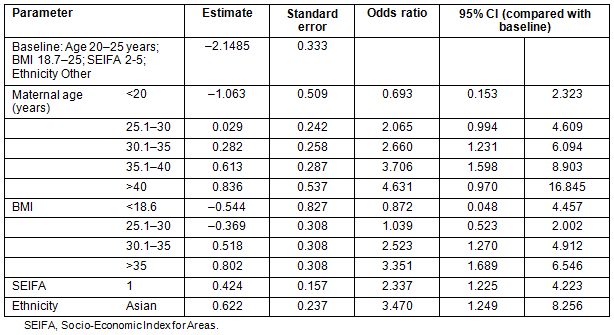
Receiver-operator characteristic (ROC) curves were plotted for the model as a whole and for individual parameters (Fig1). The area under the curve was calculated as 0.706 (standard error 0.034). The model was 68.0% concordant in this population.
Table 3 gives the details of the comparison of ROC curves calculated from the different models, each variable on its own, maternal age and BMI together and the full model. Although SEIFA and ethnicity do not have strong effects, they are still significant at 0.05 and thus retained as part of the final model.
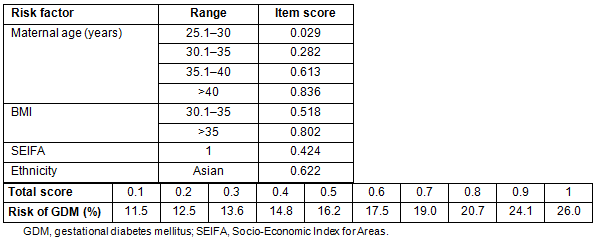
The scoring system using the factors remaining significant in the model is shown in Table 4. Age <20 years and BMI of underweight and overweight were not significantly different from baseline so were not scored as separate items. The total score is estimated by adding the individual item scores for each woman, then interpolating the risk from the right-hand side of the table and from the bottom of the table. Thus, a Caucasian woman aged 32 with a BMI of 32 but SEIFA class 4 has a total score of 0.282+0.518=0.8, giving a risk of 20.7% for developing GDM.
The best discriminating score was 0.4, giving a sensitivity of 69.9%, and a specificity of 61.2%. Although the positive predictive value at this cut-off was only 7.8%, the negative predictive value, which is more important, was 97.8%. It should be noted that maternal age >40 years, BMI>30, SEIFA class 1 or Asian ethnicity alone identify the woman as having at least a one in six risk for developing gestational diabetes.
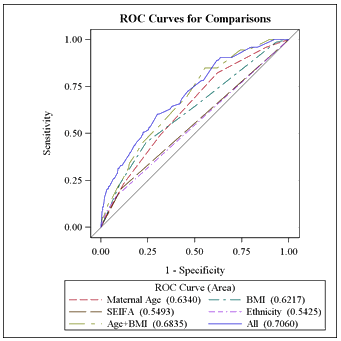
Figure 1: Receiver-operator characteristic curves for comparisons.
Table 3: Associations for receiver-operator characteristic curves
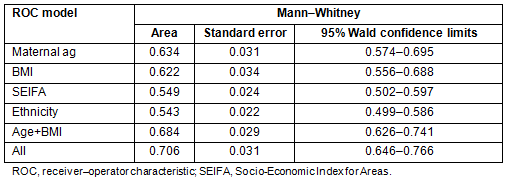
Table 4: The gestational diabetes mellitus scoring system
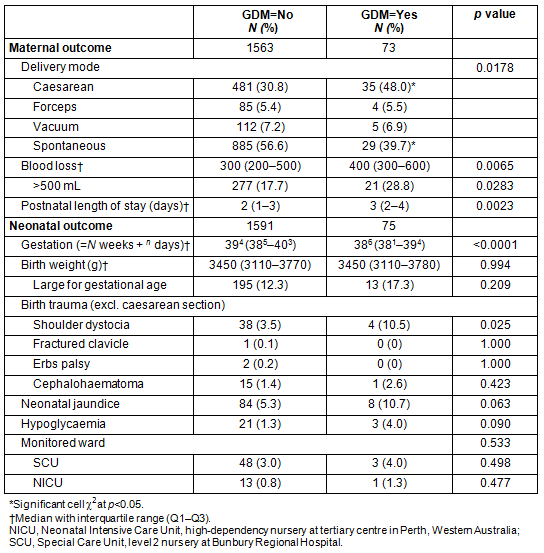
The effect of GDM in pregnancy on maternal and neonatal outcomes is shown in Table 5. Birth outcomes were analysed using Fisher's exact test due to low numbers. The maternal outcomes information shows there is a significant effect of having GDM in this population on delivery mode (p=0.0178), with a significantly higher rate of caesarean section and fewer spontaneous births. These women had a higher blood loss overall (KW test, p=0.0065) and a higher proportion with greater than 500 mL loss, the threshold definition of postpartum haemorrhage (p=0.0114). Mothers with GDM had a longer median length of stay in hospital (2 vs 3 days; KW test, p=0.0023). Mothers who had GDM gave birth with a median difference in gestation of 5 days earlier (KW test, p=0.0001) but there was no difference in the birth weight of the infants, nor for the proportion that were large for gestational age (corrected for gestation). The infants of the mothers who had GDM had a threefold higher rate of shoulder dystocia (p=0.025) but there were no other differences.
Table 5: Effect of gestational diabetes mellitus on maternal and neonatal outcomes
Discussion
The authors believe this is the first published review of GDM in an Australian rural regional centre. The study presents two years of obstetric data for BRH, a referral hub for south-west WA. In particular, the incidence of GDM was shown to be similar to that found in the state overall. Within the population, there were subgroups at higher risk. These groups included the obese, older mothers and those in the lowest socioeconomic quintile. Specific ethnic groups were not shown to be at higher risk within this population except for Asian mothers. There was no independent effect for ATSI women, a surprising finding as other studies8 have shown that this group is at increased risk for gestational diabetes and for type 2 diabetes. This result may have been due to low population numbers, with only 5.3% of the population being in this racial group. Alternatively, there may have been a referral effect, with the highest risk patients being referred out of Bunbury to a tertiary centre in Perth. The data to explore this possibility were not available for this study.
While the strong association of obesity with GDM is no surprise, the number of pregnant women who were overweight or obese is a concern. In the study population, less than half were in the normal weight range and a quarter were obese. Clearly, obesity is a major concern in this population. The lowest socioeconomic quintile had over twice the rate of GDM of the rest of the population. However, the association of the SEIFA quintile 1 with GDM was statistically independent of the effect of obesity. This implies that low socioeconomic status in itself is a predictive factor for GDM. This is consistent with similar findings elsewhere9. In terms of a target group, the obese, and in particular the lowest socioeconomic strata, are the women who would benefit the most from intervention. Women with GDM present an increased care burden for BRH, with longer hospital stays, increased maternal haemorrhage, increased shoulder dystocia and increased surgical intervention.
This study has a number of limitations. First, it records data from one institution. It is not truly representative of the whole obstetric population as there are other hospitals in the region delivering babies. Unfortunately, access to the obstetric data from the other hospitals was not readily available. While two years of data yielded a sample size with over 95% power to identify twofold changes in probability with five covariates, when broken into subgroups, for some, such as ethnicity, the sample sizes were too small to make broader generalisations. The absence of increased risk for GDM in the ATSI ethnic group is a finding differing from other studies8. This may reflect the small sample size, a sampling bias (with severely affected patients being referred to a tertiary centre) or something different about the ATSI patients in this community.
This is the first study to look at what is happening with regards to GDM within a rural regional WA hospital. The catchment of BRH covers a wide spectrum of socioeconomic status, including areas of relative affluence. Elsewhere in rural WA, there are regions with significantly lower socioeconomic status, such as the Kimberley, which may have a much higher risk of GDM. The results of this study suggest a comparative study of antenatal risk factors for GDM in other rural regions is required.
Universal screening for GDM has been recommended for all women at 26-28 weeks gestation for some time17 and has been recently updated18. However, it is still important to identify those women at higher risk of GDM to allow earlier intervention or additional screening outside the recommended protocol. Using the simple scoring system presented in this paper, rural practitioners may be able to distinguish between those women who have a 98% chance of not developing GDM and those for whom extra vigilance may identify those with the condition, allowing earlier intervention and improving maternal and neonatal outcomes.
These results confirm the known association of GDM with age; obesity, lower socioeconomic quintile and Asian ethnicity are also present in this rural population. The authors failed to find an association of GDM risk with Aboriginal ethnicity. This was not expected and possible reasons for this are discussed. A predictive model is demonstrated that can accurately identify for this obstetric population those at lowest risk of developing GDM.
Acknowledgements
The Rural Clinical School of Western Australia funded this project. Western Australian Country Health Service provided access to the Stork database. Karen Hanson prepared the data set for analysis. David Atkinson and Kirsten Auret reviewed the manuscript. Linda Hemson and Maureen Hutchinson provided assistance with access to the data.
References
1. Joyce A, Tran BN. Western Australian mothers and babies, 2009: twenty-seventh annual report of the Western Australian Midwives' Notification System. Department of Health Western Australia, 2011.
2. Landon MB, Spong CY, Thom E et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009; 361(14): 1339-1348.
3. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP et al. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008; 358(19): 1991-2002.
4. Crowther CA, Hiller JE, Moss JR et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005; 352(24): 2477-2486.
5. Gee V. Perinatal, infant and maternal mortality in Western Australia. 2004-2008. Perth, WA: Department of Health, 2010.
6. Janus E, Laatikainen T, Dunbar J et al. Overweight, obesity and metabolic syndrome in rural southeastern Australia. Medical Journal of Australia 2007; 187(3): 147-152.
7. Cleland V, Hume C, Crawford D et al. Urban-rural comparison of weight status among women and children living in socioeconomically disadvantaged neighbourhoods. Medical Journal of Australia 2010; 192(3): 137-140.
8. Stone C, McLachlan K, Halliday J et al. Gestational diabetes in Victoria in 1996: incidence risk factors and outcomes. Medical Journal of Australia 2002; 177(9): 486-491.
9. Vibeke A, van der Ploeg HP, Cheung NW, Huxley RR, Bauman AE. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care 2008; 31(12): 2288-2293.
10. Berkowitz G, Lapinski R, Wein R et al. Race/ethnicity and other risk factors for gestational diabetes. American Journal of Epidemiology 1992; 135(9): 965-973.
11. Smith JP. Nature and causes of trends in male diabetes prevalence, undiagnosed diabetes, and the socioeconomic status health gradient. Proceedings of the National Academy of Science USA 2007; 104(33): 13 225-13 231.
12. Roberts C, Lancaster P. Australian national birthweight percentiles by gestational age. Medical Journal of Australia 1999; 170: 114-118.
13. Australian Bureau of Statistics. SEIFA: socio-economic indexes for areas. Available: www.abs.gov.au/websitedbs/D3310114.nsf/home/Seifa_entry_page (accessed August 2012).
14. Australian Bureau of Statistics. 2003.0.55.001 - Census of Population and Housing: Socio-economic Indexes for Areas (SEIFA), Data only 2006. (Online) 2008. Available: www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/2033.0.55.0012006?OpenDocument (Accessed August 2012).
15. Martin F, Vogue A, Dargaville R, Ericksen C, Oats J, Tippet C. The diagnosis of gestational diabetes. Medical Journal of Australia 1991; 155: 112.
16. Sullivan LM. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Statistics in Medicine 2004; 23(10): 1631-1660.
17. Hoffman L, Nolan C, Wilson Dennis J, Oats Jeremy JN, Simmons D. Gestational diabetes mellitus - management guidelines. The Australasian Diabetes in Pregnancy Society. Medical Journal of Australia 1998; 169(2): 93-97.
18. Nankervis A, McIntyre HD, Moses R, Ross GP, Callaway L, Porter C et al. Australasian Diabetes in Pregnancy Society (ADIPS) consensus guidelines for the testing and diagnosis of gestational diabetes mellitus in Australia. Sydney, NSW: Australasian Diabetes in Pregnancy Society, 2013.

