full article:
Chronic pain is a major public health problem that affects an estimated 116 million Americans and is one of the most common reasons for individuals to seek healthcare services1. It was recently reported by the Institute of Medicine that chronic pain costs approximately $635 million annually. Most of this cost is due to increased healthcare utilization and lost productivity1. A majority of patients with chronic pain seek treatment from multiple providers and endure several surgical procedures in order to gain some relief2. This places an enormous burden on community healthcare resources as well as being a drain on the individual's emotional, physical, and financial resources. There is empirical evidence that psychological distress, pain severity, and pain-related disability predict healthcare utilization in patients with chronic pain3-6. It is noteworthy, however, that most of these studies have been conducted in large urban multispecialty hospitals. Given that urban and rural social structures and lifestyles differ significantly, it is possible that the factors affecting health services use by people living in rural areas may diverge from those in urban areas7. It is thus surprising that there is a paucity of research focused on healthcare use in underserved rural populations living with chronic pain. It is logical to assume that in rural areas, high utilization of health services by individuals with chronic pain may multiply the burden on an already limited resource pool, leading to insufficient pain relief and frustration for both the patient and the provider.
Rural residency is associated with higher prevalence of chronic pain and other psychiatric and medical comorbidities, especially depression8,9. Rural residents with chronic pain report higher pain frequency and intensity, as well as more pain-related disability and depression than people with pain living in urban areas7,10. Healthcare disparities between rural and urban areas are widely recognized, and residents of rural areas often experience difficulties related to availability, accessibility, and affordability of health services11. This additionally puts rural patients with chronic pain at a higher risk for adverse health outcomes.
Currently, opioids are being increasingly prescribed for non-malignant chronic pain12-15. Many practitioners believe that opioids are more efficacious in providing rapid relief to individuals who are not finding relief with other analgesics for their persistent non-malignant pain13. A recent epidemiological study examining the trends of prescription analgesics indicated that 45.7% of people who reported chronic pain were prescribed narcotic analgesics16. Indeed, at present, opioids are one of the most widely prescribed analgesics for chronic pain in the USA17. With this increasing use, there is considerable concern about the addiction potential of opioids as well as their association with increased mortality and various negative health outcomes, especially with long-term use18. Furthermore, there are increasing concerns about opioid-induced hyperalgesia19,20 . This refers to a paradoxical condition wherein a patient receiving opioid analgesics experiences heightened sensitivity to pain, in relation to the underlying pain condition as well as new pains.
Previous research suggests a relationship of receiving opioid prescription on a long-term basis with psychosocial variables, including psychological distress, pain-related disability, and a history of substance abuse21,22. It is surprising though that the role of pain intensity or other pain variables such as duration or type of pain in prescription of these drugs remains inconclusive23. Merrill and colleagues found that patients on chronic opioid therapy were more likely to endorse psychosocial distress as well as meet criteria for clinical depression24. Indeed, some authors have postulated that long-term opioid use may actually be leading to or exacerbating depression25. Conversely, it is possible that the presence of depressive or distress symptoms in patients with chronic pain influences the providers' decision to prescribe opioids (considered the 'stronger' of the analgesic options). This may especially be so in geographical areas (eg medically underserved rural counties) where multidisciplinary treatment options are limited and opioids may be considered as the best of a very limited range of options for patients with chronic pain and depression. Hence, it would be prudent that appropriate psychological evaluations are completed prior to the initiation of opioids and regularly thereafter26.
The non-medical use of prescription opioids in rural areas has recently gained attention and is becoming a reason for concern27,28. A large US-based pharmacoepidemiology study comparing urban, suburban, and rural areas in their use and misuse of prescription opioids indicated that the rates of abuse of prescription opioids in rural areas was disproportionately higher29. A recent article indicated that the rate of deaths due to drug overdoses within the rural areas in the USA has now surpassed those in the urban areas30. The authors have attributed these trends to the rapidly escalating rates of prescription of drugs such as opioids in rural areas. This has led to increased accessibility and availability of addiction-forming drugs in rural areas where street drugs such as heroin are not readily available30. Given such grave concerns, it becomes vital to gain insight into the patterns of opioid prescription in rural counties. It is important to identify the individuals who are more likely to receive such prescriptions as well as the related psychosocial variables that are potentially modifiable. Such information may help inform alternative interventions that can be employed to lower psychological distress and pain while enhancing health and wellness.
The primary aim of the present study was to explore the association of demographic, pain, and psychosocial variables with the overall healthcare use in a 3 month period in patients with chronic pain living in a rural area. These patients were receiving care at Federally Qualified Health Centers (FQHC) in two medically underserved counties of a south-eastern state in the USA. Psychosocial and demographic measures at baseline were used as predictors. Based on previous literature, the influence of pain intensity, psychological distress, pain catastrophizing, pain-related disability, and prescription opioids on healthcare use was examined. Although empirical support for the influence of demographic factors on healthcare use is inconsistent, the association of age, sex, and ethnicity with health services use was also explored in this study. As a secondary aim, the influence of these same demographic and psychosocial variables on the prescription of opioids was analyzed.
Participants and recruitment sites
The present analyses were based on a subset of participants recruited from FQHCs serving low-income patients in rural Alabama, and who were enrolled for a larger upcoming randomized controlled clinical trial comparing cognitive behavioral therapy31 to an education intervention32 for chronic pain. Patients above 19 years of age and experiencing pain for most days in a month over the past 3 months were eligible to participate. Medical records of 64 out of 106 participants in the larger study were available for review (60.4%); the rest were not medical patients at the sites where the psychosocial interventions were to be conducted. Demographic, pain and psychosocial characteristics are shown in Table 1. The participants were recruited from Wilcox and Walker counties, Alabama. Both of these counties have been classified as medically underserved areas33.
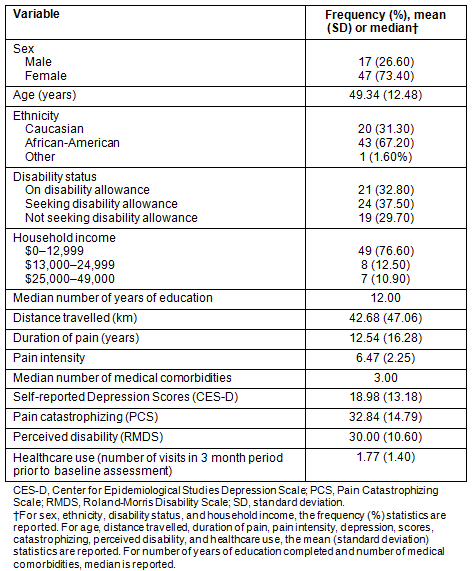
Table 1: Demographics, pain characteristics, and psychosocial measures (N=64)
Procedure
Participants were recruited by healthcare providers, community flyers and patient word-of-mouth. Demographic and psychosocial measures were collected as part of the pretreatment assessment. Informed consent was obtained at the pretreatment interview. Medical records were reviewed on-site by a trained doctoral student, who is also a physician. Participants received compensation for travel expenses and for time and effort spent on the baseline assessment.
Measures
Demographics: The demographic questionnaire, developed for this study, was used to collect information about age, ethnicity, sex, disability status, education, relationship status, and annual household income. In addition, accessibility to the healthcare center was calculated in terms of total distance travelled to reach the healthcare center.
Structured pain interview: This was adapted with permission to determine patient report of type(s) of pain, location(s) of pain, and primary pain type and site34. The interview also helped to distinguish any conditions that might contraindicate participation in the study, such as pain associated with malignant disease (ie cancer pain, HIV pain)35.
Pain intensity and interference: Using the Wisconsin Brief Pain Inventory, participants rated their worst pain, least pain, and average pain over the past week, as well as their current pain levels on a 10 point Likert scale36. The participants also rated interference due to pain in their daily activities, mood, and sleep. The Brief Pain Inventory has demonstrated good internal consistency in a variety of pain populations37. The overall internal consistency for this sample as measured by Crohnbach's alpha was 0.93.
Psychosocial measures
Catastrophizing: The Pain Catastrophizing Scale (PCS) was used to measure pain catastrophizing38. The PCS is a 13 item measure asking respondents to rate the extent to which they have particular thoughts when they experience pain. The PCS measures catastrophizing on three dimensions, namely magnification, rumination and helplessness, and the total score for catastrophizing is the sum of the raw scores. Higher scores indicate greater catastrophic thinking. The internal consistency for this sample measured by Crohnbach's alpha was 0.95.
Depression: The Center for Epidemiological Studies Depression Scale (CES-D) was used to assess symptoms of depression39. It is a 20 item questionnaire in which respondents rate the frequency with which each item occurred over the previous week. Higher scores indicate greater depressive symptoms. The CES-D has high internal consistency, adequate test-retest reliability, and convergent as well as discriminant validity. The CES-D has been validated for use in patients with chronic pain40. The internal consistency for this sample measured by Crohnbach's alpha was 0.90.
Perceived disability: The Roland-Morris Disability Scale (RMDS), an 11 item scale was used to measure perceived disability due to pain41. A total score was obtained by summing the number of items endorsed (from 0 to 11). The RMDS is the most widely used scale for assessing treatment outcomes in pain management programs in terms of reduction in perceived disability41. The internal consistency for this sample as measured by Crohnbach's alpha was 0.80.
Healthcare utilization: Healthcare use data were obtained retrospectively from manually maintained (hand-written) medical records of the participants and included the total number of visits in a period of 3 months prior to baseline. The purpose of the visits was multifaceted and included pain management as well as acute illness and chronic comorbidity management. Data pertaining to prescription analgesics, including opioids, as well as medical and psychiatric comorbidities, were also recorded.
Data analysis
The data were analyzed using IBM SPSS Statistics v19.0 (www-01.ibm.com/software/analytics/spss). Descriptive analyses of demographics, pain characteristics, psychosocial measures, and healthcare utilization are reported as means and corresponding standard deviations or as frequency and corresponding percentages. Preliminary correlational analyses were conducted to examine association of the demographic characteristics (age, sex), pain variables (intensity, interference), number of medical comorbidities, and psychosocial variables (self-reported disability due to pain, depression, and catastrophizing) with the number of visits in a 3 month period prior to the psychosocial interventions. The number of visits was used as the variable measuring healthcare use. A Poisson regression model was utilized to analyze the variables affecting healthcare use. This was done as the utilization data collected for this study are count data. The demographic and psychosocial variables significantly associated with number of visits were entered as independent variables in the regression model with the number of visits as the dependent variable.
To examine the factors associated with the prescription of opioids, preliminary χ² analyses (categorical independent variables) as well as correlational analyses (continuous independent variables) were conducted. Binomial logistic regression was conducted to analyze the predictors of receiving an opioid prescription based on the significant associations in the preliminary analyses. Potential violations of linearity, multicollinearity, or homoscedasticity were assessed in each analysis.
Ethics approval
This study was approved by the Institutional Review Board at the University of Alabama, Tuscaloosa (approval number IRB# 07-003-ME).
Participants
The participants were rural residents in medically underserved counties, primarily female (73.4%), African-American (67.2%), and approximately 77% reporting annual household income of less than $13,000. Approximately 70% were either on disability allowances or seeking disability allowances. The mean self-reported pain intensity was 6.47 on a scale of 1 to 10. For further description about demographics, pain variables, and healthcare use, see Table 1. For information on medical comorbidities, see Table 2.
Table 2: Medical comorbidities

Recruitment sites
Both of the recruitment counties have been classified as medically underserved areas31. County-specific census data, as provided by the US Census data 2010, were similar to the study sample in terms of race (Wilcox: χ²=0.90, p>0.90; Walker: χ²=1.60, p>0.05)31. However, the number of females in this sample was higher (χ²=12.26, p<0.05), which is not surprising given that more women than men suffer from or self-report chronic pain42,43.
Healthcare utilization
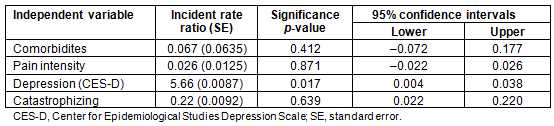
Preliminary bivariate analyses indicated that depressive symptoms and pain catastrophizing were the only two variables significantly associated with healthcare use. There was no association of health services use with age, sex, ethnicity, opioid prescriptions, distance from the healthcare center, or perceived pain-related disability (Table 3). Self-reported depression scores and pain catastrophizing scores were entered in the Poisson regression model. Since the number of comorbidities and pain intensity were important variables of interest in regard to the frequency of visits to the healthcare center, they were entered on the first step of the Poisson regression model and the depression and catastrophizing were added next. The omnibus test indicated that only the depression scores obtained from CES-D uniquely and significantly predicted the number of visits in the 3 month period. Higher levels of self-reported depression scores (incidence rate ratio (IRR)=5.66, p=0.017, 95% confidence intervals between 0.004 and 0.038) were associated with greater number of visits (Table 4).
Table 3: Correlation between demographic, psychosocial variables, number of visits pretreatment, and opioid prescription
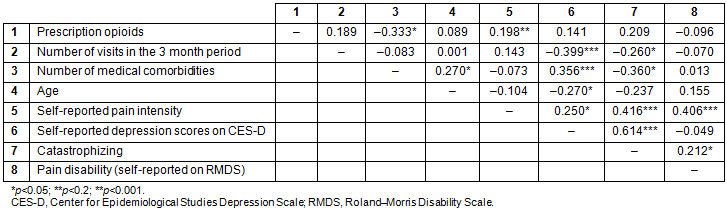
Table 4: Poisson regression for the association of healthcare utilization with pain intensity,
number of comorbidities, self-reported depression scores (CES-D), and pain catastrophizing scores (N=64)
Prescription opioids
Approximately 40% (n=26) of the participants received opioid prescription in the 3 month period included in the chart review. The rest received prescription non-steroidal anti-inflammatory drugs (NSAIDs; n=38; 59.4%). A χ² analysis indicated a near-significant trend of males being approximately three times more likely to receive a prescription of opioids than females (χ²=3.513, p=0.05, odds ratio (OR)=2.99). In addition, the patients with a clinical diagnosis of depression based on chart review were 3.4 times more likely to get opioids (χ²=4.608, p=0.032, OR=3.42). The association of race or age with receiving a prescription of opioids was non-significant. Similarly, there was no association with pain intensity, pain interference, perceived pain-related disability, and pain catastrophizing with receiving an opioid prescription.
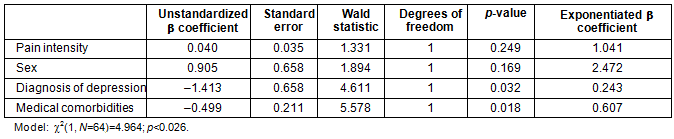
Based on the significant and near significant associations (0.05-0.2) as indicated by the preliminary bivariate correlation analyses, sex, pain intensity, number of medical comorbidities, and the diagnosis of clinical depression were entered in the listed order to the binary logistic model examining the predictors of receiving an opioid prescription. The results indicated that a clinical diagnosis of depression as well as the number of medical comorbidities significantly and uniquely predicted opioid prescriptions (χ²(1, N=64)=4.964; p<0.026). Pain intensity and sex did not contribute significantly to the model (Table 5).
Table 5: Binary logistic regression predicting opioid prescription (N=64)
Discussion
The present study is one of the first to focus on healthcare use in low-income rural patients with chronic pain living in medically underserved areas. This provides a rare examination of a unique and understudied population with gross disparities in treatment access and health outcomes. The results suggest that higher scores on self-reported depressive symptoms were associated with greater health services use. This is consistent with previous literature that shows psychological distress is associated with increased healthcare use in patients with chronic illnesses, including chronic pain, and has important clinical implications44,45. The results highlight the need for healthcare providers to evaluate symptoms of depression in patients presenting with chronic pain in rural FQHCs, especially as access to specialized mental health services in such locations is extremely restricted46.
An important finding of the study suggests that 40% of the participants were prescribed opioids for their chronic pain. This finding is consistent with epidemiological studies examining the proportion of individuals with chronic pain taking prescription opioids47. It is noteworthy that those with a clinical diagnosis of depression were more than three times likely to receive opioid prescriptions for their chronic pain. The results are consistent with the suggested role of depression and psychological distress in the likelihood of receiving opioid therapy. Similar results have been found in different clinical populations with non-malignant chronic pain, including veterans with chronic pain, patients with back pain and patients experiencing whiplash injuries48,49. It is plausible that individuals with higher pain levels are experiencing heightened distress and receive more opioid medication. A recent survey examined concerns and problems of patients with non-cancer chronic pain on long-term opioid therapy related to their prescription drugs. The results suggested that patients who were prescribed higher opioid doses reported slightly higher pain intensity and pain-related impact on life than those on lower doses24. In addition, patients who were on higher doses reported higher levels of clinically diagnosed depression. It is plausible then that opioids increase the risk of psychiatric illness, thus exacerbating the problem. In this context, there may be additional variables, such as opioid-induced hyperalgesia that contribute towards exacerbating psychological distress19. On the other hand, it may be that in rural FQHCs with limited treatment options, distress and depression may be cues for finding a 'stronger' medication option (such as opioids). Additionally, it is possible that the participants of the present study had poorly managed pain, leading to heightened psychological distress.
There is evidence that among individuals with no substance abuse history, those who are clinically diagnosed with depression and prescribed opioids for their pain are more likely to misuse prescription opioids than those who are not50. Indeed, it has been reported that individuals with coexistent psychiatric and medical comorbidities such as cardiovascular disorders are at a greater risk for prescription opioid abuse51. Given that a high number of the participants in the present study had comorbid depression (approximately 30%) and medical comorbidities (approximately 86%), opioid prescription is all the more concerning. Furthermore, given that the participants of the present study are medically underserved, it is possible that such FQHCs are the most or only accessible source of health care. Thus, it would be beneficial for the primary healthcare providers at such low-income rural healthcare centers to routinely evaluate a patient with chronic pain for associated psychological distress and mental health status, especially prior to initiating opioid therapy. Offering and providing psychosocial treatments, such as adapted cognitive behavioral interventions, could prove helpful in reducing the need for opioids52,53.
There were limitations to the present study. The visits were cumulative of all the visits made to the community care center and not specifically pain-related visits. Furthermore, it is possible that the reported pain intensity, pain-related disability, and opioid prescriptions were additionally influenced by the level of service availability as well as rurality. These data were not available for the present study, hence could not be analyzed. However, it would be useful to analyze these potential variables in the future. Although data pertaining to self-reported depression as well as a diagnosis of depression were collected and analyzed, additional data were not available for other psychiatric comorbidities, such as anxiety, which may have influenced the participants' psychological distress. The sample size was restricted due to the limited availability of medical records at the health centers. Also, due to limited resources, it was not possible to conduct fidelity checks to ensure the accuracy of medical records. For future studies, it would be worthwhile to formally assess other types of healthcare visits, including emergency visits and visits to specialists in rural people with pain to see how much these outlets meet the health services needs of this underserved population.
Pain in rural areas is an extremely understudied area of research. Patients with pain living in rural areas are often underserved and poor31. They report more pain severity and co-occurring psychological distress with lower or limited access to healthcare services than individuals with chronic pain living in urban areas7. The study highlights the association of psychological distress with healthcare use in rural patients. In addition, the patients who were diagnosed with depression and those with a greater number of medical comorbidities were more likely to be prescribed stronger painkillers, opioids, notwithstanding the pain severity. This emphasizes the need to integrate medical and psychological care for such patients at rural healthcare centers. Psychosocial interventions may provide a feasible and cost-effective source of health care that may translate into decreased psychological distress and improved quality of life. Furthermore, alternative interventions for pain may help reduce the perceived need for opioid medications, thus decreasing the associated risk factors. Towards this end, the integration of mental health professionals into primary care, when enacted, may increase access to such services. Other innovative care models, such as group medical visits54 or peer-led or lay-led self-management and psychosocial intervention groups55, may be a way to penetrate limited access areas and offer at least limited services.
Acknowledgements
This research was supported by the National Institute of Nursing Research and the National Institute of Mental Health, NR010112. The authors have no conflicts of interest to report. The authors thank all of the patients and medical staff who made this research possible. The authors also acknowledge Drs Jennifer Gardner and Melissa Day for their earlier contributions to this research project.
References
1. Board on Health Sciences Policy. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: Institute of Medicine of the National Sciences. (Online) 2011. Available: www.iom.edu/Reports/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research.aspx (Accessed 31 May 2012).
2. Mayday Fund. A call to revolutionize chronic pain care in America: an opportunity in health care reform. New York: (Online) 2009. Available: www.MaydayPainReport.org (Accessed 3 October 2013).
3. Blyth FM, March LM, Brnabic AJM, Cousins MJ. Chronic pain and frequent use of health care. Pain 2004; 1(11): 51-58.
4. Engel CC, von Kroff M, Katon WJ. Back pain in primary care: predictors of high health-care costs. Pain 1996; 65: 197-204.
5. Groenewald C, Essner B, Lewandowski A, Palermo T. Predictors of health care utilization in an international treatment-seeking cohort of adolescents with chronic pain. Journal of Pain 2013; 14(4): S39-S39.
6. Lee JL, Gilleland J, Campbell RM, Simpson P, Johnson GL, Dooley KJ, et al. Health care utilization and psychosocial factors in pediatric noncardiac chest pain. Health Psychology 2013; 32(3): 320.
7. Adam P, Goode PT, Janet K, Freburger PT, Carey TS. The influence of rural versus urban residence on utilization and receipt of care for chronic low back pain. Journal of Rural Health 2012; 29(2): 205-214.
8. Tollefson J, Usher K. Chronic pain in the rural arena. Australian Journal of Rural Health 2006; 14: 134-135.
9. Rost K, Fortney J, Fischer E, Smith J. Use, quality, and outcomes of care for mental health: the rural perspective. Medical Care Research and Review 2002; 59(3): 231-265.
10. Hoffman P, Meier B, Council JR. A comparison of chronic pain between an urban and rural population. Journal of Community and Health Nursing 2002; 19(4): 213-224.
11. Nelson JA, Gingerich BS. Rural health: access to care and services. Home Health Care Management and Practice 2010; 22(5): 339-343.
12. Kalso E. Opioids in chronic non-malignant pain. ABC of Pain 2012; 218: 103.
13. Ballantyne JC, Mao J. Opioid therapy for chronic pain. New England Journal of Medicine 2003; 349(20): 1943-1953.
14. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013; 154: S94-S100.
15. Kissin I. Long-term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety? Journal of Pain Research 2013; 6: 513.
16. Toblin RL, Mack KA, Perveen G, Paulozzi LJ. A population-based survey of chronic pain and its treatment with prescription drugs. Pain 2011; 152(6): 1249-1255.
17. Laxmaiah M, Standiford H, Fellows B, Janata J, Pampati V, Grider DO, et al. Opioid epidemic in the United States. Pain Physician 2012; 15: ES9-ES38.
18. Dhalla IA, Persaud N, Juurlink DN. Facing up to the prescription opioid crisis. British Medical Journal 2011; 343: 1-4.
19. Lee M, Silverman S, Hansen H, Patel V. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011; 14: 145-161.
20. Johnson JL, Hutchinson MR, Williams DB, Rolan P. Medication-overuse headache and opioid-induced hyperalgesia: a review of mechanisms, a neuroimmune hypothesis and a novel approach to treatment. Cephalalgia 2013; 33(1): 52-64.
21. Højsted J, Ekholm O, Kurita GP, Juel K, Sjøgren P. Addictive behaviors related to opioid use for chronic pain: a population-based study. Pain 2013; 154(12): 2677-2683.
22. Andersen, T. E. Does attachment insecurity affect the outcomes of a multidisciplinary pain management program? The association between attachment insecurity, pain, disability, distress, and the use of opioids. Social Science and Medicine 2011; 74(9): 1461-1468.
23. Sullivan MD, Edlund MJ, Steffick D. Unützer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain 2005; 119(1): 95-103.
24. Merrill JO, Korff MV, Banta-Green CJ, Sullivan MD, Saunders KW, Campbell CI, et al. Prescribed opioid difficulties, depression and opioid dose among chronic opioid therapy patients. General Hospital Psychiatry 2012; 34(6): 581-587.
25. Trevino CM, deRoon-Cassini T, Brasel K. Does opiate use in traumatically injured individuals worsen pain and psychological outcomes? Journal of Pain 2013; 14(4): 424-430.
26. Leo RJ, Mariano MT. Psychological distress and psychiatric comorbidities in palliative care. In: Essentials of palliative care. N Vadivelu, AD Kaye, JM Berger (Eds). New York: Springer Science-Business Media, 2013.
27. McCabe SE, West BT, Boyd CJ. Medical use, medical misuse, and nonmedical use of prescription opioids: results from a longitudinal study. Pain 2013; 154(5): 708-713.
28. Cranford JA, Boyd CJ, McCabe SE. Adolescents' nonmedical use and excessive medical use of prescription medications and the identification of substance use subgroups. Addictive Behaviors 2013; 38(11): 2768-2771.
29. Cicero TJ, Surratt H, Inciardi JA, Munoz A. Relationship between therapeutic use and abuse of opioid analgesics in rural, suburban, and urban locations in the United States. Pharmacoepidemiology and Drug Safety 2007; 16(8): 827-840.
30. Okie S. A flood of opioids, a rising tide of deaths. New England Journal of Medicine 2010, 363(21): 1981-1985.
31. Thorn BE, Day MA, Burns J, Kuhajda MC, Gaskins SW, Sweeney K, et al. Randomized trial of group cognitive behavioral therapy compared with a pain education control for low-literacy rural people with chronic pain. Pain 2011; 152: 2710-2720.
32. Ehde D, Jensen M, Engel J, Hanley M, Raichle K, Osborne T. Education intervention therapist manual: project II: role of catastrophizing in chronic pain (unpublished manual) 2005.
33. US Department of Health and Human Services Health Resources and Services Administration. Available: http://muafind.hrsa.gov/index.aspx (Accessed 30 May 2013).
34. Blanchard EB, Andrasik F. Management of chronic headaches: a psychological approach. Elmsford, NY: Pergamon Press, 1985.
35. Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. Journal of Pain 2005, 6(3): 149-158.
36. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Academy of Medicine, Singapore 1994; 23(2): 129-138.
37. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. Journal of Pain 2004; 5(2): 133-137.
38. Sullivan, MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment 1995, 7(4): 524-532.
39. Radloff LS. The Center for Epidemiologic Studies Depression Scale: a self report depression scale for research in the general population. Applied Psychological Measurement 1977; 1(3): 385-401.
40. Turk DC, Okifuji A. Detecting depression in chronic pain patients: adequacy of self-reports. Behaviour Research and Therapy 1994; 32(1): 9-16.
41. Stroud MW, McKnight PE, Jensen MP. Assessment of self-reported physical activity in patients with chronic pain: development of an abbreviated Roland-Morris Disability Scale. Journal of Pain 2004; 5(5): 257-263.
42. Elliott AM, Smith BH, Penny KI, Cairns W, Chambers AW. The epidemiology of chronic pain in the community. The Lancet 1999; 354: 1248-1252.
43. Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Practice and Research Clinical Rheumatology 2011; 25(2): 173-183.
44. Grabe HJ, Baumeister SE, John U, Freyberger HJ, Völzke H. Association of mental distress with health care utilization and costs: a 5-year observation in a general population. Social Psychiatry and Psychiatric Epidemiology 2009; 44(10): 835-844.
45. Becker A, Held H, Redaelli M, Strauch K, Chenot JF, Leonhardt C, et al. Low back pain in primary care: costs of care and prediction of future health care utilization. Spine 2010; 35(18): 1714-1720.
46. Larrison CR, Hack-Ritzo S, Koerner BD, Schoppelrey SL, Ackerson BJ, Korr WS. Economic grand rounds: state budget cuts, health care reform, and a crisis in rural community mental health agencies. Psychiatric Services 2011; 62(11): 1255-1257.
47. Toblin RL, Mack KA, Perveen G, Paulozzi LJ. A population-based survey of chronic pain and its treatment with prescription drugs. Pain 2011; 152(6): 1249-1255.
48. Dobscha SK, Morasco BJ, Duckart JP, Macey T, Deyo RA. Correlates of prescription opioid initiation and long-term opioid use in veterans with persistent pain. Clinical Journal of Pain 2013; 29(2): 102-108.
49. Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. Journal of Pain 2003; 4(6): 344-350.
50. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Annals of Family Medicine 2012; 10(4): 304-311.
51. Katz C, El-Gabalawy R, Keyes KM, Martins SS, Sareen J. Risk factors for incident nonmedical prescription opioid use and abuse and dependence: results from a longitudinal nationally representative sample. Drug and Alcohol Dependence 2013; 132(1-2): 107-113.
52. Turk D. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clinical Journal of Pain 2002; 18(6): 257-263.
53. Kuhajda MC, Thorn BE, Gaskins SW, Day MA, Cabbil CM. Literacy adaptations for cognitive behavioral therapy in a rural pain population. Translational Behavioral Medicine 2010; 1(2): 216-223.
54. Miller D, Zantop V, Hammer H, Faust S, Grumbach K. Group medical visits for low-income women with chronic disease: a feasibility study. Journal of Women's Health 2004; 13(2): 217-225.
55. Griffiths C, Foster G, Ramsay J, Eldridge S, Taylor S. How effective are expert patient (lay led) education programmes for chronic disease? BMJ 2007; 334(7606): 1254-1256.

