full article:
In the USA, people living in rural areas report poorer health status than urban residents1-5. This health disparity is often linked to rural residents' lack of access to healthcare services, shortages of healthcare professionals, lower rates of health insurance, problems working with third-party payers, and low socioeconomic and educational status6-21. For example, individuals living in rural areas experience a higher prevalence of chronic diseases such as obesity, heart disease, cancer, and diabetes than people in urban areas2,21-26.
US states like South Carolina (SC), with large rural populations, are particularly vulnerable to rural-urban health disparities, with rural residents facing poorer overall health, engaging less in healthy behaviors, and lacking access to preventive services and care for chronic diseases such as diabetes27. Nearly 35% of the population in South Carolina lives in rural areas (Fig1)27,28. This represents more than 1.5 million people living in small and remote locations across the state who may be lacking needed medical care28.
A possible solution for providing health care to these medically underserved communities is through clinical trials (CTs)29,30. Clinical trials are biomedical or health-related research studies intended to add to medical knowledge31. CTs can offer patients the most advanced medical treatments and screening options, and can help participants such as those in rural areas obtain the medical care they need30,31. Despite potential 'therapeutic misconceptions', where potential disadvantages related to CT participation are overlooked32, CTs can help medically underserved populations access novel medical care and reduce health disparities33,34. Nevertheless, CT participation is particularly low among medically underserved communities (ie people living in rural areas)6,9-11,18,19,34-37.
A significant reason for low participation in CTs among rural communities is the lack of access to healthcare facilities27,38,39. The low socioeconomic status of rural individuals may also make them more sensitive to additional costs and time often required in CT participation40,41. As some trials may not be covered by insurance, some individuals may not be able to afford to participate7,8,18,42,43. In South Carolina, 25 of 46 counties are designated as medically underserved areas (MUAs) (Fig1)27. The median per capita income in 2005 for rural counties in the state was $23,344, about $11,000 less than the national average of $34,47127.
Another key barrier to CT accrual in rural areas is limited knowledge and awareness about CTs42-47. The study by Friedman et al48 on knowledge and perceptions of CTs found that rural residents share important misperceptions about CTs, which may be fueled by some lack of knowledge and understanding. Further, Lara et al46 found a correlation between education level and knowledge about CTs, with the highly educated being more knowledgeable and willing to participate in medical research. According to US Census data, nearly 30% (29.4%) of adults in rural South Carolina lack a high school diploma49. These less educated rural individuals may be less knowledgeable about CTs50,51 and, therefore, less likely to participate.
Although CT participation is low in rural areas18,35,52, no study has explored the proportion of rural residents who are eligible to participate in CTs. CT participants must meet specific inclusion criteria to qualify for a study. These criteria vary by trial, and are often based on factors such as age, gender, race, health insurance status, type and stage of disease, previous treatment history, and other medical conditions30,31,53.
Pre-existing medical conditions based on a clinical risk grouping (CRG), a health-status classification system often utilized by insurance companies and government-funded programs54, are another important exclusion criterion for participation in CTs39,53. Depending on the CT, an individual may not be allowed to participate if he or she has multiple comorbidities and disabilities.
Regardless of the type of trial, CT principal investigators (PIs) are ultimately responsible for accruing patients into their CTs. To the authors' knowledge, there are no studies that assess what CT PIs think about their accrual of patients in rural and urban areas or studies that explore PIs' perceived barriers to CT participation. The aim of this study is to address this gap in the literature by examining the perceptions of PIs regarding CT participation barriers and recruitment efforts in rural areas of South Carolina. A secondary data analysis of eligible South Carolina CT participants was also conducted to assess the potential pool of CT participants in rural and urban areas of South Carolina. The ultimate goal was to compare PIs' perceptions of eligible participants to the actual pool of participants.
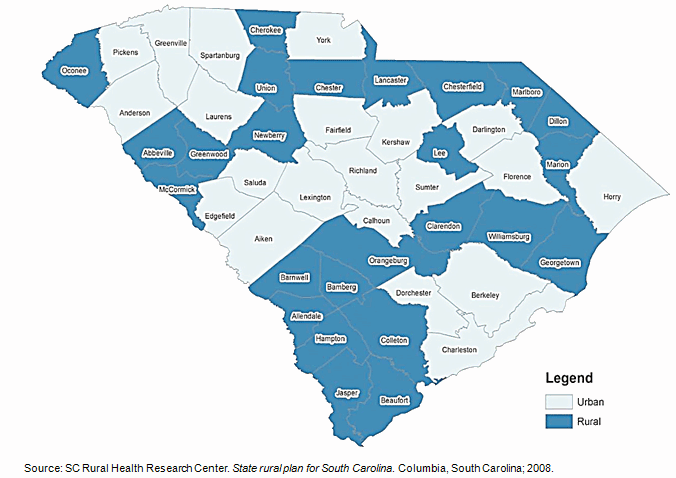 Figure 1: Rural and urban counties in South Carolina, USA27.
Figure 1: Rural and urban counties in South Carolina, USA27.
This study used both a survey and secondary data analysis to examine CT participation and eligibility. First, an online survey was developed and used to assess the PIs' perceptions about CT participation. PIs at the five large primary research hospitals in South Carolina were approached because the trials conducted at these institutions are systematically documented and conducted by South Carolina-based researchers.
Next, analyses of existing data from government programs like South Carolina Medicaid, the South Carolina State Health Plan (SHP), and the US Census, allowed the authors to describe a number of household- and individual-level characteristics of importance to CT eligibility in South Carolina. Medicaid provides an important source of insurance coverage for medically underserved populations55. In South Carolina, 8% of people in the state, or more than 350 000 individuals, are insured by Medicaid, including citizens in rural areas. Since rural populations are frequently categorized among the medically underserved, Medicaid data were used for these analyses.
Another 445 000 people in South Carolina are covered by the SHP (10%) (D. Dickerson, pers. comm., 2011). As individuals covered by the SHP are often considered representative of the state's population, for the purposes of this research these data were extrapolated to the rest of the non-Medicaid population (A. Brock Martin, pers. comm., 2013). The SHP, coupled with Medicaid, covers 18% of the state's population for health care (not included are other programs such as Medicare-age 65+ (5%), Veterans Affairs (VA) health insurance (8%), other private insurance (55%) for which data were unavailable, and the uninsured (17%) (D Dickerson, pers. comm., 2011)56. While this is a limitation of the present study, the representative nature of the SHP data helps validate results. Finally, the census data were used to complement the Medicaid and SHP data for specific criteria relating to clinical trial participation (eg education and language).
Principal investigator survey
An e-survey using Qualtrics, a subscription-based survey website for researchers in industry and academia (http://www.qualtrics.com), was administered to PIs at South Carolina's five main academic medical centers located in urban areas of the state: Spartanburg Regional Healthcare System and Gibbs Cancer Center, Greenville Hospital System University Medical Center, Medical University of South Carolina, University of South Carolina and University of South Carolina School of Medicine, and Palmetto Health Hospital. The survey was open for 3 weeks during June and July of 2011. The survey link was sent to 382 potential survey participants identified through a variety of tracking methods (see Tanner et al57 for detailed information on survey development and data collection). A convenience sample of 119 CT investigators completed the survey, for a response rate of 31%58. Respondents could only click and access the survey once, which prevented the same person from completing the survey multiple times59. The majority (83%) of respondents were PIs, while the others were project managers, recruiters, research associates, nurses, or 'other'. Because of their work with CTs, all respondents were included in this study and are referred to as PIs throughout this article.
The survey instrument was composed of 33 items, which were developed based on extensive literature on CT participation6,8,9,12,36,43,46,47. The survey instrument included a combination of open- and closed-ended questions to gather information about the volume and scope of CT research taking place at South Carolina's main academic medical centers, the extent to which rural residents in South Carolina are represented in clinical research, and PIs' perceptions of barriers to CT recruitment. All responses were captured automatically via the website59. The survey instrument was approved by the university's Institutional Review Board.
Although the survey, as a whole, addressed a wide range of issues pertaining to CT participation and recruitment, for the current study the researchers focused on several variables that specifically aligned with the secondary data. These six items touched on PIs' perceptions of the barriers to CT participation in the general population and in rural communities, and included:
- difficulty finding potential clinical trial participants
- patients' lack of awareness and knowledge about CTs
- lack of literacy
- limited access to trial sites
- lack of insurance coverage
- lack of information about available trials.
For each item, respondents were asked to rate their level of agreement on a five-point Likert scale that ranged from 'strongly disagree' to 'strongly agree'. No incentives were provided to survey respondents. As described elsewhere57, parametric statistics including paired-sample t-tests were used to analyze the survey data. Analyses were generated using the Statistical Package for the Social Sciences v20 (SPSS Inc.; http://www-01.ibm.com/software/analytics/spss/products/statistics).
Secondary data analysis
Three data sets were used to assess the CT eligibility of rural South Carolina populations. First, data sets describing South Carolina Medicaid recipients and those covered by the SHP were used. Data were provided by the South Carolina Office of Research and Statistics (ORS; http://ors.sc.gov). Individuals in these data sets were grouped by age and CRGs by county. The Medicaid data set compiled information on the population of South Carolina 18 years and older insured by Medicaid, excluding the optional coverage for women and infants (OCWI). The SHP data set compiled information on the population of South Carolina 18 years and older insured by the SHP.
Next, data from the US Census (2005-2009) American Community Survey (ACS) were used and compiled at the individual and household levels. While some of these data are available online (http://www.census.gov/acs/www), data sets were received for education, language, poverty level, and access to a vehicle through the ORS. The rationale for the assessment of these variables is explained in Figure 2.
It is important to point out that each of these data sets (Medicaid, SHP, ACS) reports data differently. For example, the ACS data included both individual-level (education, language, and poverty level) and household-level data (access to a vehicle). For the purpose of this research, some variables were collapsed to represent 'eligible' and 'not eligible' based on the defined set of criteria. For example, education was reported in five categories from 'no high school diploma' to 'graduate degree'. Based on the determined eligibility criterion for education (high school diploma or above), this became a dichotomous variable, with percentage 'no high school' versus percentage 'high school and above'.
Language was reported in a set of categories that focused on populations that speak English, Spanish, Indo-European languages, Asian and Pacific Island languages, and other languages 'well' or 'very well'. For this variable, those who speak English 'well' and 'very well' were collapsed with or without other languages versus all those who do not speak English well.
Access to a vehicle was reported in the ACS data as having 'no access to a vehicle' versus 'access to one or more vehicles' per household. For this variable, the categories were kept intact and excluded individuals aged 65 or older. Regarding poverty level, only those whose income was above federal poverty standards were included in this study as it was assumed that they would have access to health insurance or could cover the additional costs related to the CTs60.
In the SHP and Medicaid data sets, CRGs are divided into levels 1-9. Insurance companies, including government-funded programs, such as Medicaid, often classify the health status of individuals into CRGs, which assign each individual to a single mutually exclusive risk group based on clinical (ie pre-existing medical conditions) and demographic characteristics54. For this study, individuals in CRG levels 1-5 are considered as potentially eligible CT participants. Individuals assigned to these groups are one of the following:
- healthy (CRG 1)
- have a history of significant acute disease (CRG 2)
- have a single minor chronic disease (CRG 3)
- have a minor chronic disease in multiple organ systems (CRG 4)
- have a single dominant or moderate chronic disease (CRG 5).
Levels 1-9 nine were therefore divided into two categories - eligible (CRG 1-5: healthy or may have up to one single dominant or moderate chronic disease), and ineligible (CRG 6-10: having comorbidities). Medicaid also categorized individuals by 'disability' or 'no disability'. For Medicaid, individuals who were categorized as having a disability were excluded from the analyses.
The age criterion was reported in each of the three data sets as follows: 18-24, 25-34, 35-44, 45-54, 55-64, and >65 years. For the purposes of this study, only age groups between 18 and 64 years, based on general age requirements for CT participation, were included.
Because the focus was on rural populations specifically, the secondary data were divided by urban and rural regions. As the data were reported by county, each county could be designated as primarily urban or rural, based on ORS designations. There are a number of definitions of 'rural' because important differences, including demographic, cultural, and economic ones, exist across and among rural places61. For this research, an urban or metropolitan area is defined as an area with at least 50 000 people, while a rural area can be a micropolitan, a small rural or small remote rural area composed of 50 000 people or less27. Finally, for the secondary data analyses, descriptive statistics, frequencies, and paired-sample t-tests were generated using Excel 2007 and SPSS. For the t-tests, the assumption of normality was assessed for the dependent variable and was not violated.
 Figure 2: Rationale for selecting the following four American Community Survey (ACS) variables
Figure 2: Rationale for selecting the following four American Community Survey (ACS) variables
Ethics approval
All components of this study were approved by the university's Institutional Review Board; ethics approval number Pro0009909.
Principal investigator survey
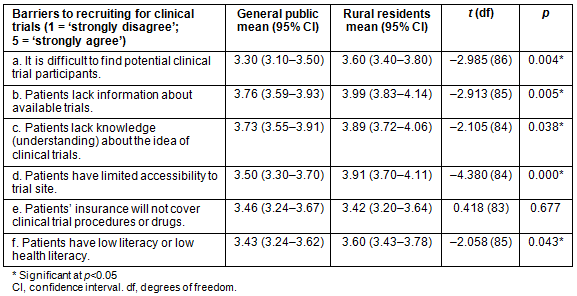
As shown in Table 1, respondents perceived the barriers to recruitment to be far greater in rural areas than in the general population. Principal investigators indicated that finding potential clinical trial participants was significantly more difficult in rural areas than in the general population (t=-2.985, p=0.004). In terms of education about CTs, rural residents were significantly more likely to be perceived as lacking knowledge and understanding about the idea of CTs than the general public (t=-2.105, p=0.038), which may affect their participation in medical research. Additionally, rural residents were perceived to have significantly lower literacy or lower health literacy than the general public (t=-2.058, p=0.043), which can be a barrier to CT recruitment and participation.
In rural communities, lack of information about available trials was found to be significantly more likely to be a barrier to CT participation than in the general population (t=-2.913, p=0.005). Access was also a barrier that was considered for CT participation across the state. Patients in rural areas were also significantly more likely to be perceived as having limited accessibility to trial sites compared to the general population (t=-4.380, p=0.000). This may be because fewer CTs are held in rural areas, thus requiring patients to have access to a vehicle to participate. Additionally, insurance coverage was examined as a barrier to CT participation. According to PIs, patients' insurance coverage of CT procedures or drugs was not considered more of a barrier for rural residents than for the general public (t=0.418, p=0.677). In other words, insurance coverage may not hinder CT participation in rural areas more than in urban areas of the state.
Table 1: Perceived barriers to clinical trial participation by population (general public, rural residents)
Secondary data analysis
A secondary data analysis of Medicaid, SHP, and ACS was conducted to assess whether or not the perceptions of the PIs regarding barriers to CT participation in rural communities were indeed the reality in rural South Carolina. Forty-five percent of Medicaid recipients live in rural areas and 41% of people insured by the SHP live in rural areas. When looking at CT eligibility by CRG level, Medicaid data revealed that the proportion of rural and urban residents with a CRG level between 1 and 5, or relatively healthy individuals with no comorbidities, is nearly equal. Based on these results, when looking at CRG level alone, 70% of individuals living in rural areas insured by the SHP or Medicaid are eligible for CTs.
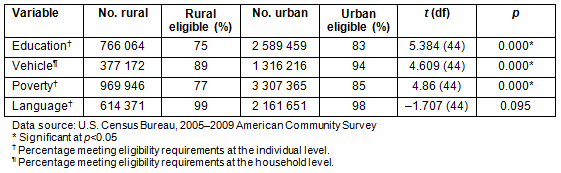
The ACS data, which showed differences between urban and rural citizens on variables such as education, access to a vehicle, poverty, and language, were analyzed to compare these numbers to PI perceptions on lack of knowledge and information about CTs (eg relates to education level and language), accessibility to trial site (eg relates to access to vehicle and poverty level), low literacy (eg relates to education and language). As shown in Table 2, a greater percentage of South Carolina residents have a high school diploma or higher in urban areas (83%) compared to rural areas (75%), and this difference is statistically significant (t=5.384, p=0.000). The data also indicate that more individuals live above poverty level in urban areas (85%) compared to rural areas of the state (77%) (t=4.86, p=0.000). Poverty, therefore, affects more people in rural areas of South Carolina. Eighty-nine percent of citizens in rural areas have access to a vehicle compared to 94% in urban areas, and this difference was also statistically significant (t=4.609, p=0.000). The difference in terms of language (speaking English well or very well) is not statistically significant between rural and urban areas of the state (t=-1.707, p=0.095). While it was not possible to obtain the percentage of individuals in rural areas of South Carolina who meet all eligibility criteria at the same time (ie who have a high school diploma or higher, who own a vehicle, who live above poverty level, and who speak English well or very well) because of the way the data were presented, it was possible to get a better picture of CT eligibility. Based on these analyses, a minimum of 75% of individuals in rural areas meet at least one of these CT eligibility criteria, compared to 83% or more of individuals in urban areas of the state.
Table 2: Estimated rural and urban eligibility for clinical trials
Discussion
This study examined both sides of a situation not previously studied: PIs' perceptions of CT barriers among rural residents (survey research) and the proportion of potentially eligible participants in rural areas of the state (secondary data analyses). These analyses revealed similarities and differences between PIs' perceptions and potential participant eligibility.
Principal investigators indicated that it is most difficult to find eligible CT participants in rural areas of the state. Although the overall analyses of existing data revealed that fewer eligible participants live in rural areas of South Carolina than in urban areas (75% vs 83%), there still exists a large, untapped pool of eligible participants in rural South Carolina. With low levels of CT participation one of the most significant challenges facing medical research8,62, these findings suggest that rural populations represent a real opportunity for participant recruitment. Further, CTs may be an important alternative for individuals in underserved medical communities to access effective medical care30,31. More than one million people living in rural areas of the state could access quality medical care while contributing to medical research and scientific knowledge though their participation in CTs30,31,33,34.
As emphasized by results from the PI survey and secondary analyses, however, there are barriers, as perceived by respondents in the PI survey and found to be a reality in the demographic data, that hinder CT enrollment in rural communities. Accessibility to trial sites, for example, remains a barrier for CT participation in rural areas. PIs believed that rural residents have limited accessibility to trial sites compared to the general public and the secondary data suggest that rural residents may have slightly less access to a vehicle to get to the CT site (89%) than their urban counterparts (94%) (t=4.609, p=0.000). Further, because more people live in poverty in rural areas than in urban areas of South Carolina (23% vs 15%, respectively), this may affect their ability to pay for a clinical trial, have access to health insurance, purchase a vehicle, or travel to a trial site.
Principal investigators also believed that rural residents are more likely to lack knowledge and understanding about the idea of CTs than the general public. Although there was not a direct connection to 'knowledge and understanding' in the secondary data, it was possible to assess educational attainment and language. One quarter of residents in rural South Carolina lack a high school diploma, nearly 15% more than those living in urban areas of the state. On the other hand, language does not seem to be a barrier, as the data indicated that 99% of rural residents speak English well or very well. Specifically, findings suggest that rural citizens may speak the language used in clinical trials but may not understand the complicated medical jargon. This may decrease their comfort level with the idea of medical research and ultimately prevent them from enrolling in a CT50,51,63,64 .
Interestingly, PIs' perceptions of insurance coverage by population, rural versus urban, were in line with the demographic data. Clinical trial PIs perceived little variance between urban and rural populations on insurance coverage. The Medicaid and SHP data also revealed that South Carolina residents' insurance coverage is nearly equal in rural and urban areas (70% vs 72%, respectively).
This comparison of PIs' perceived barriers to CT participation in rural South Carolina to the actual characteristics of this population represents the first attempt to assess investigators' level of understanding of their target population. This study suggests that PIs, who were recruited from South Carolina's main academic medical centers located in urban areas, may be perpetuating unhelpful rural myths about CT eligibility in rural communities65. Despite their remote locations, rural citizens should also take part in medical research, as it can play an important role in improving their health66.
Education should be provided to CT PIs to inform them about this large pool of potentially eligible CT participants in rural South Carolina and to correct their misperceptions about CT eligibility. PI training should also be available to help PIs improve their CT recruitment efforts in rural areas of the state. Different strategies could be used to address the different barriers to CT participation and increase CT recruitment in rural areas, including providing plain language CT information, offering transportation to the trial sites, and providing free medical care as incentives to participate67,68
A few limitations affect the generalizability of these findings. For the PI survey, respondents were only recruited from South Carolina's five main academic medical centers; PIs conducting CTs from pharmaceutical companies and other settings were not included. The authors are confident, however, that this sample included the majority of PIs conducting CTs in South Carolina. In addition, the response rate of 31% for the PI survey may be considered low in research. However, this rate is fairly typical for online surveys with this specific population69-72. The total number of responses per question on the PI survey varied considerably. The survey, which was presented in an online format where participants could 'quit' the survey at any time, may have been too long for PIs, which created some participant burden73-75. Finally, the closed-ended survey items may not have been the most appropriate data collection method to extensively investigate the PIs' perceptions of CTs. The response options ('strongly agree' to 'strongly disagree') limited the collection of detailed comments from the PIs. Future research should consider using qualitative methods to explore the PIs' perceptions of clinical trial recruitment barriers.
For the secondary data analysis, individuals without health insurance or with private insurance were not included. While this limits the portrayal of CT eligibility in the state, the analyses conducted using ACS, Medicaid, and SHP data still provided a comprehensive overview of participant eligibility. Moreover, because of the way raw data were presented (individual and household levels), it was not possible to build on the current findings by combining all ACS data, or even combining the Medicaid and the SHP data sets to obtain a more complete representation of CT eligibility in South Carolina. Therefore, a final percentage of those potentially eligible for CTs and those who are not could not be obtained. Lastly, knowledge and education as they are used in the PI survey were not explicitly defined and categorized as they are in the secondary data analysis. Nevertheless, there was still a match between PIs' perceptions about lower levels of knowledge and education about CTs among rural residents and lower educational attainment in rural areas, as suggested by the secondary data.
This study was the first step in trying to provide a comprehensive representation of CT eligibility in South Carolina. It was also the first step in examining the state of CT recruitment among rural populations and the barriers to CT participation. PIs who participated in this study acknowledged the barriers they face in recruiting CT participants from rural areas. Variables examined for the secondary data analysis were aligned with these barriers, but this analysis also suggested that a large pool of eligible CT participants does reside in rural South Carolina.
Investigators' poor understanding of communities' perceptions about CTs often leads them into a practice of planning for rather than planning with underserved communities, resulting in poor CT participation in these communities76. Findings from the current study suggest that investigators should tailor CT educational and promotional material for rural residents by taking into account their lower educational attainment and income levels. Further, developing satellite trial sites, located in remote and rural communities, would allow those without access to a vehicle, or money for long-distance travel, an opportunity to participate in potentially life-saving research. Future research should focus on working with these rural communities to understand their medical and communication needs, as well as providing communication training to PIs to improve CT recruitment efforts in rural areas of the state.
Acknowledgments
This research was funded by Health Sciences South Carolina. The authors are grateful to Dr Jay Moskowitz from Health Sciences South Carolina and Dr Amy Brock Martin from the South Carolina Rural Health Research Center for their continuous support and advice during this project as well as providing some important contextual information on health insurance in the state. Thanks to Dennis Dickerson, Heather Kirby, and Amanda Murphy from the South Carolina Office of Research and Statistics for sharing their datasets, maps of South Carolina, as well as answering questions during the secondary data analysis.
References
1. Schorr V, Crabtree DA, Wagner D, Wetterau P. Difference in rural and urban mortality: implications for health education and promotion. Journal of Rural Health 1989; 5(1): 67-80.
2. Bennett K, Olatosi B, Probst J. Health disparities: a rural-urban chartbook. (Online) 2008. Available: http://rhr.sph.sc.edu/report/(7-3)%20Health%20Disparities%20A%20Rural%20Urban%20Chartbook%20-%20Distribution%20Copy.pdf (Accessed 18 February 2013).
3. Goode AP, Freburger JK, Carey TS. The influence of rural versus urban residence on utilization and receipt of care for chronic low back pain. Journal of Rural Health 2012, spring; 29(2): 205-214.
4. Krishna S, Gillespie KN, McBride TM. Diabetes burden and access to preventive care in the rural United States. Journal of Rural Health 2010; 26(1): 3-11.
5. Befort CA, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the United States: findings from NHANES (2005-2008). Journal of Rural Health 2012; 28(4): 392-397.
6. Baquet CR, Commiskey P, Mullins D, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detection and Prevention 2006; 30(1): 24-33.
7. Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Annals of Epidemiology 2000; 10(8 Suppl): S13-S21.
8. Brown DB, Topcu M. Willingness to participate in clinical treatment research among older African Americans and Whites. Gerontologist 2003; 43(1): 62-72.
9. Christian MC, Trimble EL. Increasing participation of physicians and patients from underrepresented racial and ethnic groups in National Cancer Institute-sponsored clinical trials. Cancer Epidemiology, Biomarkers & Prevention 2003; 12(3): 277s-283s.
10. Ford JG, Howerton MW, Bolen S, Gary TL, Lai GY, Tilburt J et al. Knowledge and access to information on recruitment of underrepresented populations to cancer clinical trials. Evidence Report/Technology Assessment (Summary) 2005; June(122): 1-11.
11. Ford ME, Havstad SL, Tilley BC. Recruiting older African American men to a cancer screening trial (the AAMEN Project). Gerontologist 2003; 43(1): 27-35.
12. Frank G. Current challenges in clinical trial patient recruitment and enrollment. Society for Clinical Research Associates Source 2004; 2: 30-38.
13. Friedman DB, Corwin SJ, Rose ID, Dominick GM. Prostate cancer communication strategies recommended by older African American men in South Carolina: a qualitative analysis. Journal of Cancer Education 2009; 24(3): 204-209.
14. Health Sciences South Carolina. Rural health care. (Online) 2013. Available: http://www.healthsciencessc.org/rural.asp (Accessed 15 December 2012).
15. Moinpour CM, Atkinson JO, Thomas SM, Underwood SM, Harvey C, Parzuchowski J et al. Minority recruitment in the prostate cancer prevention trial. Annals of Epidemiology 2000; 10(8 Suppl): S85-S91.
16. Pinto HA, McCaskill-Stevens W, Wolfe P, Marcus AC. Physician perspectives on increasing minorities in cancer clinical trials: an Eastern Cooperative Oncology Group (ECOG) Initiative. Annals of Epidemiology 2000; 10(8 Suppl): S78-S84.
17. Probst JC, Moore CG, Glover SH, Samuels ME. Person and place: the compounding effects of race/ethnicity and rurality on health. American Journal of Public Health 2004; 94(10): 1695-1703.
18. Sateren WB, Trimble EL, Abrams J, Brawley O, Breen N, Ford L et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. Journal of Clinical Oncology 2002; 20(8): 2109-2117.
19. Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Annals of Epidemiology 2002; 12: 248-256.
20. Smith KB, Humphreys JS, Wilson MGA. Addressing the health disadvantage of rural populations: How does epidemiological evidence inform rural health policies and research? Australian Journal of Rural Health 2008; 16: 56-66.
21. Gamm L, Hutchison L. Public health rural health priorities in America: where you stand depends on where you sit. Journal of Rural Health 2003; 19(3): 209-213.
22. Gamm L, Hutchison L, Bellamy G. Rural healthy people 2010: identifying rural health priorities and models for practice. Journal of Rural Health 2002; 18(1): 9-14.
23. Southwest Rural Health Research Center. Rural Healthy People 2010: A companion document to Healthy People 2010. (Online) 2010. Available: http://srph.tamhsc.edu/centers/rhp2010 (Accessed 1 February 2013).
24. Monroe AC, Ricketts TC, Savitz LA. Cancer in rural versus urban populations: a review. Journal of Rural Health 1992; 8(3): 212-220.
25. Ricketts TC. Rural health in the United States. New York, NY: Oxford University Press, 1999.
26. Wright JS, Champagne F, Dever GE, Clark FC. A comparative analysis of rural and urban mortality in Georgia, 1979. American Journal of Preventive Medicine 1985; 1(1): 22-29.
27. SC Rural Health Research Center. State rural plan for South Carolina. Columbia, South Carolina: SC Rural Health Research Center, 2008.
28. U.S. Census Bureau. 2010 Census urban and rural classification and urban area criteria. (Online) 2013. Available: http://www.census.gov/geo/www/ua/2010urbanruralclass.html (Accessed 15 December 2012).
29. Mayer-Davis EJ, D'Antonio AM, Smith SM, Kirkner G, Levin Martin S, Parra-Medina D et al. Pounds off with empowerment (POWER): a clinical trial of weight management strategies for black and white adults with diabetes who live in medically underserved rural communities. American Journal of Public Health 2004; 94(10): 1736-1742.
30. Robertson N. Clinical trial participation: viewpoints from racial/ethnic groups. Paper presented at National Conference on Clinical Trials. Atlanta, GA, 1994.
31. National Institutes of Health. Learn about clinical studies. (Online) 2012. Available: http://www.clinicaltrials.gov/ct2/info/understand#Q01 (Accessed 1 December 2012).
32. Appelbaum PS, Roth LH, Lidz CW, Benson P, Winslade W. False hopes and best data: consent to research and the therapeutic misconception. Hastings Center Report 1987; 17(2): 20-24.
33. Paskett ED, Reeves KW, McLaughlin JM, Katz ML, McAlearney AS, Ruffin MT et al. Recruitment of minority and underserved populations in the United States: the centers for population health and health disparities experience. Contemporary Clinical Trials 2008; 29(6): 847-861.
34. Guadagnolo BA, Petereit DG, Helbig P, Koop D, Kussman P, Dunn EF et al. Involving American Indians and medically underserved rural populations in cancer clinical trials. Clinical Trials 2009; 6(6): 610-617.
35. Cohen GI. Clinical research by community oncologists. CA: A Cancer Journal for Clinicians 2003; 53(2): 73-81.
36. Giuliano AR, Mokuau N, Hughes C, Tortolero-Luna G, Risendal B, Ho R et al. Participation of minorities in cancer research: the influence of structural, cultural, and linguistic factors. Annals of Epidemiology 2000; 10(8 Suppl): S22-S34.
37. Royal C, Baffoe-Bonnie A, Kittles R, Powell I, Bennett J, Hoke G et al. Recruitment experience in the first phase of the African American Hereditary Prostate Cancer (AAHPC) study. Annals of Epidemiology 2000; 10(8 Suppl): S68-S77.
38. Paskett E, Cooper MR, Stark N, Ricketts TC, Tropman S, Hatzell, T et al. Clinical trial enrollment of rural patients with cancer. Cancer Practice 2002; 10(1): 28-35.
39. Murthy V, Krumholz H, Gross C. Participation in cancer clinical trials: race-, sex-, and age-based disparities. Journal of the American Medical Association 2004; 291(22): 2720-2726.
40. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer 2005; 103(3): 483-491.
41. McCabe MS, Varricchio CG, Padberg RM. Efforts to recruit the economically disadvantaged to national clinical trials. Seminars in Oncology Nursing 1994; 10(2): 123-129.
42. Comis RL, Miller JD, Aldige CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. Journal of Clinical Oncology 2003; 21(5): 830-835.
43. Klabunde CN, Springer BC, Butler B, White MS, Atkins J. Factors influencing enrollment in clinical trials for cancer treatment. Southern Medical Journal 1999; 92(12): 1189-1193.
44. Go RS, Frisby KA, Lee JA, Mathiason MA, Meyer CM, Ostern JL et al. Clinical trial accrual among new cancer patients at a community-based center. Cancer 2006; 106(2): 426-433.
45. Lara PN, Higdon R, Lim N, Kwan K, Tanaka M, Lau DH et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. Journal of Clinical Oncology 2001; 19(6): 1728-1733.
46. Lara PN, Paterniti DA, Chiechi C, Turrell C, Morain C, Horan N et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. Journal of Clinical Oncology 2005; 23(36): 9282-9289.
47. Meropol NJ, Buzaglo JS, Millard J, Damjanov N, Miller SM, Ridgway C et al. Barriers to clinical trial participation as perceived by oncologists and patients. Journal of the National Comprehensive Cancer Network 2007; 5(8): 753-761.
48. Friedman DB, Bergeron CD, Foster C, Tanner A, Kim SH. What do people really know and think about clinical trials? A comparison of rural and urban communities in the South. Journal of Community Health 2013; 38(4): 642-651.
49. Rural Policy Research Institute. Data & resources - state profile. (Online) 2009. Available: http://www.rupri.org/dataresearchviewer.php?id=6 (Accessed 15 December 2012).
50. Miller JD. Scientific literacy in the United States. Communicating science to the public. In: D Evered D, M O'Connor (Eds). Communicating science to the public. London: Wiley, 1987; 19-40.
51. Miller JD, Pardo R, Niwa F. Public perceptions of science and technology: A comparative study of the European Union, the United States, Japan, and Canada. Madrid: BBV Foundation Press; 1997.
52. Maurer LH, Davis T, Hammond S, Smith E, West P, Doolittle M. Clinical trials in a rural population: professional education aspects. Journal of Cancer Education 2001; 16(2): 89-92.
53. Van Spall H, Toren A, Kiss A, Fowler R. Eligibility criteria of randomized controlled trials published in high-impact general medical journals. Journal of the American Medical Association 2007; 297(11): 1233-1240.
54. Hughes JS, Averill RF, Eisenhandler J, Goldfield N, Muldoon J, Neff JM et al. Clinical risk groups (CRGs): a classification system for risk-adjusted capitation-based payment and health care management. Medical Care 2004; 24(1): 81-90.
55. Rowland D, Lyons B, Rudowitz R. Medicaid as a platform for broader health reform: supporting high-need and low-income populations. (Online) 2009. Available: http://www.dhcs.ca.gov/provgovpart/Documents/Waiver%20Renewal/Medicaid%20for%20Broader%20Health%20Reform.pdf (Accessed 1 December 2012).
56. Kaiser Family Foundation. Health coverage & uninsured. (Online). Available: http://www.statehealthfacts.org/comparecat.jsp?cat=3&rgn=42&rgn=1 (Accessed 1 December 2012).
57. Tanner A, Kim SH, Friedman DB, Foster C, Bergeron CD. Barriers to medical research participation as perceived by clinical trial investigators: communicating with rural and African American communities. Journal of Health Communication 2013 (in press).
58. Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. International Journal for Quality in Health Care 2003; 15(3): 261-266.
59. Eysenbach G. Improving the quality of web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). Journal of Medical Internet Research 2004; 6(3): e34.
60. U.S. Census Bureau. American Community Survey and Puerto Rico Community Survey: 2011 subject definitions. (Online) 2011. Available: http://www.census.gov/acs/www/Downloads/data_documentation/SubjectDefinitions/2011_ACSSubjectDefinitions.pdf (Accessed 15 December 2012).
61. Hart G, Larson E, Lishner D. Rural definitions for health policy and Rpresearch. American Journal of Public Health 2005; 95(7): 1149-1155.
62. Collins FS. From the NIH Director: the importance of clinical trials. NIH Medline Plus 2011; 6(2) (Online). Available: http://www.nlm.nih.gov/medlineplus/magazine/issues/summer11/articles/summer11pg2-3.html (Accessed 15 December 2012).
63. Comis RL, Crowley J, Unger J, Marinucci D, Catalano R, Colaizzi DD et al. Baseline study of patient accrual onto publicly sponsored U.S. cancer clinical trials: an analysis conducted for the global access project of the National Patient Advocate Foundation. Philadelphia, PA: Coalition of Cancer Cooperative Groups, 2006; 1-52.
64. Atkinson NL, Saperstein SL, Massett HA, Leonard CR, Grama L, Manrow R. Using the Internet to search for cancer clinical trials: a comparative audit of clinical trial search tools. Contemporary Clinical Trials 2008; 29(4): 555-564.
65. Kapferer JL. Rural myths and urban ideologies. Journal of Sociology 1990; 26(1): 87-106.
66. Lionis C, Tatsioni T. Conducting research in rural primary care medicine: do we need more experimental research or guidance? Rural and Remote Health 12: 2267 (Online) 2012. Available: www.rrh.org.au (accessed 29 October 2013).
67. Friedman DB, Hoffman-Goetz L. A systematic review of readability and comprehension instruments used for print and Web-based cancer information. Health Education & Behavior 2006; 33(3): 352-373.
68. Owens OL, Jackson DD, Thomas TL, Friedman DB, Hebert JR. African-American men's and women's perceptions of clinical trials research: focusing on prostate cancer among a high risk population in the South. Journal of Health Care for the Poor and Underserved 2013; 24(4): 1784-1800.
69. Braithwaite D, Emery J, de Lusignan S, Sutton S. Using the Internet to conduct surveys of health professionals: a valid alternative? Family Practice 2003; 20(5): 545-551.
70. Jepson C, Asch DA, Hershey JC, Ubel PA. In a mailed physician survey, questionnaire length had a threshold effect on response rate. Journal of Clinical Epidemiology 2005; 58(1): 103-105.
71. Kellerman S, Herold J. Physician response to surveys: a review of the literature. American Journal of Preventive Medicine 2001; 20(1): 61-67.
72. Knapp H, Kirk S. Using pencil and paper, Internet and touch-tone phones for self-administered surveys: does methodology matter? Computers in Human Behavior 2003; 19(1): 117-134.
73. Porter SR, Whitcomb ME, Weitzer WH. Mulitiple surveys of students and survey fatique. New Directions for Institutional Research 2004; 121: 63-73.
74. Galesic M, Bosnjak M. Effects of questionnaire length on participation and indicators of response quality in a web survey. Public Opinion Quarterly 2009; 73(2): 349-360.
75. Couper MP, Traugott MW, Lamias MJ. Web survey design and administration. Public Opinion Quarterly 2001; 65(2): 230-253.
76. Vesey GA. A successful strategy for recruitment and retention of Black Elders in applied research. African American Research Perspectives 2002; 8(2): 40-56.




