full article:
Infectious diarrhoea is common in children. Globally, it is the third leading cause of mortality in children less than 5 years, accounting for 800 000 deaths annually1. In the Northern Territory, Australia, Indigenous children are disproportionately burdened, with higher rates of hospitalisation and longer duration of illness than non-Indigenous children2. Prolonged and recurrent episodes of diarrhoeal illness can result in environmental enteropathy syndrome, and complications including severe dehydration, acidosis and hypokalaemia are more common3. Diarrhoeal episodes often compound the problem of co-existing malnutrition, risking sub-optimal growth and poor cognitive outcomes4. Rehydration and correction of electrolyte imbalances are the mainstays of treatment, with an occasional role for targeted antimicrobial therapy.
Nitazoxanide is a thiazolide antimicrobial developed in the 1980s with reported activity against a broad spectrum of pathogens. These include protozoa (Cryptosporidium, Giardia, Isospora, Cyclospora), helminths (Ascaris, Trichuris, Ancylostoma duodenale, Enterobius, Strongyloides, Taenia coli and Hymenolepis nana)5, some bacteria (bacteroides and clostridium)6, and viruses (rotavirus, respiratory syncytial virus and possibly hepatitis C)4. In vitro studies indicate nitazoxanide acts by inhibiting pyruvate-ferredoxin oxidoreductase (PFOR) enzyme-dependent reactions necessary for anaerobic energy metabolism. Nitazoxanide has also been shown to inhibit viral replication4.
Nitazoxanide is not currently licensed for use in Australia, but is recognised as an orphan drug (medicine designed to treat a rare disease or one for which treatment is not commercially available) by the Therapeutic Goods Administration and is available for compassionate use7.
There is only one retrospective study of nitazoxanide use in Australia. This was conducted by Yeung et al8 in Alice Springs, Northern Territory, between November 2006 and June 2009. The group attempted to retrospectively identify case-control subjects for the 46 Indigenous children admitted with Cryptosporidium infection and treated with nitazoxanide over the study period. Nutritional outcomes were compared at discharge and 7 months follow-up. Weight gain occurred in a higher proportion of the nitazoxanide-treated group at discharge (82% of 44 treated children had a positive change in weight for height compared to 75% of 61 untreated children) and 7 months post-discharge (66% compared to 57%). However, the significance of these results was not clear, and confounding factors such as nutritional rehabilitation and comorbidities were not considered.
A number of small, randomised control trials indicate faster recovery among children treated with nitazoxanide compared to placebo for Giardia, amoebiasis, Cryptosporidium, rotavirus, norovirus, and also for diarrhoea where no enteropathogens were found4,9-14. Using Medline, PubMed, Cochrane Library (1960-September 2011; search terms nitazoxanide, diarrhoea, Cryptosporidium, paediatric), two systematic reviews, 12 randomised control trials, and one case report were found relating to nitazoxanide use in both immunocompromised and immunocompetent children. Only articles pertaining to immunocompetent children are considered here.
The broad range of enteropathogens where efficacy has been shown raises the prospect of empiric use of nitazoxanide therapy in the treatment of undifferentiated paediatric infectious diarrhoea.
Nitazoxanide has been prescribed intermittently for children at the Royal Darwin Hospital (RDH), Northern Territory, since 2006. However, the frequency and indications for its use are not clear. The objective of this study was to review the compassionate use of nitazoxanide in this setting, and to better define the indications for, and outcomes associated with, nitazoxanide use in this setting.
The RDH is a 350-bed tertiary facility that serves the Top End (northernmost part) of the Northern Territory. This includes a population of 230 000 (49 000 ≤13 years) in an area of 516 945 km2, with 126 000 residing in Darwin. Approximately 30% of the population is Indigenous15. Pharmacy records were used to identify children aged ≤13 years prescribed nitazoxanide at the RDH from 1 July 2007 to 31 March 2012. The discharge medical diagnoses for these patients, based on the International Classification of Diseases, 10th edition (ICD-10) criteria were also recorded16. The total number of admissions to the paediatric infectious ward for the study period, including those with infectious diarrhoea, was identified.
Medical files of patients who received nitazoxanide during the audit period were reviewed by the primary investigator. Information for each participant was recorded on standardised data collection sheets, including demographics, symptoms, investigations and treatment details. Immunisation status was ascertained from the Northern Territory Immunisation Register based on adherence to the recommended Northern Territory schedule at the time of admission17. 'Remote residence' was defined as per definitions set by the Australian Bureau of Statistics18.
Nutritional status was assessed based on pre-morbid 'well weights' from health centre records prior to admission (within the previous 6 months). If a pre-morbid weight was not available, the discharge weight was used instead. World Health Organization (WHO) definitions were used to define those moderately underweight (weight-for-age z score between −3 and −2) or severely underweight (weight-for-age z score less than −3), and moderately (height-for-age z score between −3 and −2) or severely stunted (height-for-age z score less than −3)19.
Diarrhoea was defined as ≥3 liquid stools per day. Resolution of diarrhoea was defined as ≤2 stools per day, maintained for at least 72 hours or until hospital discharge. Eosinophilia was defined according to the RDH pathology reference ranges for eosinophilia at various ages.
Descriptive statistical analysis was performed using STATA v12.1 (http://www.stata.com) and Microsoft Excel Solver.
Ethics approval
Ethics approval was obtained from the Human Research Ethics Committee of the NT Department of Health and Families and Menzies School of Health Research; reference number HREC-2012-1754.
Patient details and demographics
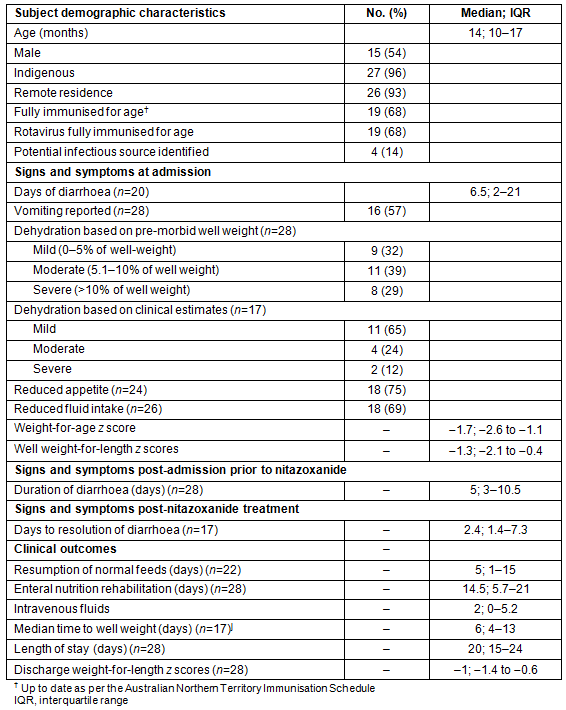
Of the 7010 admissions to the paediatric infectious ward over the study period, 1275 (18%) were for infectious gastroenteritis, and nearly three-quarters (73%) of infected children were Indigenous, mostly retrieved from remote settings. Twenty-eight children received treatment with nitazoxanide. Of these, 27 were infected with Cryptosporidium. A total of 102 children were admitted with Cryptosporidium infection during the study period, but treatment only occurred in 26% of cases. All but one of the treated children were Indigenous. The ages of treated children ranged from 4 months to 6 years (Table 1), including nine children less than 12 months old. Fifty-four per cent of the study cohort was male and <8% resided in urban areas. One-third were not fully immunised, and for the majority (86%) infectious contacts were not identified (Table 1).
Table 1: Demographics, signs and symptoms and clinical outcomes of children treated
for Cryptosporidium infection at Royal Darwin Hospital, 1 July 2007 - 31 March 2012 (n=28)
Co-existing acute medical diagnoses
An overwhelming majority of children (26) had one or more medical comorbidities. Malnutrition was common. At admission, eight children (30%) were moderately underweight, three (11%) were severely underweight, four (14%) were moderately stunted, and another four (14%) children were severely stunted. Other diagnoses included ear infections (n=11; 39%); lower respiratory tract infections (n=9; 32%); anaemia (n=6; 21%) and cutaneous fungal infections (n=6; 21%). Seven children (28%) had eosinophilia on full blood examination. Scabies and urinary tract infections were documented in a further 15% of the study cohort.
Symptoms and signs
Where data were recorded (n=20), a median of four episodes of diarrhoea occurred per day at admission, with diarrhoeal illness extending for a median of 11.5 days (6.5 days pre- and 5 days post-admission) before nitazoxanide was commenced. Vomiting, dehydration, reduced appetite and decreased fluid intake were also common among treated children (Table 1).
Diarrhoeal aetiology
Cryptosporidium was identified by microscopy in the stools of 27 (96%) nitazoxanide-treated children. One child was treated empirically despite a negative stool result because of persistent diarrhoea and malabsorption. One child was co-infected with Salmonella.
Antimicrobial treatment
The prescribed dose of nitazoxanide varied. Nineteen children (68%) were treated according to the CDC dosing protocol20. The remaining nine children were less than 12 months (minimum age 4 months; minimum weight 4.8 kg), and received doses ranging from 50 mg to 100mg BD for 3 days. Twenty-three children received the full course. All children received empiric antihelminthic treatment with albendazole for 3 days, either prior to hospitalisation or at admission. The child co-infected with Salmonella did not receive treatment other than nitazoxanide and albendazole. In the 18 (64%) patients whose stools were re-examined post-treatment, none had a positive stool result for Cryptosporidium.
Clinical outcomes
Resolution of diarrhoea was achieved a median of 2.4 days after commencement of nitazoxanide (Table 1). Normal feeds were resumed a median of 5 days after commencement of nitazoxanide, and time to recovery of pre-morbid weight was 6 days. All children recorded improved weight-for-age z scores at discharge compared to admission (−1 compared to −1.3; see Table 1). Median length of stay was 20 days. No adverse effects were recorded.
Discussion
The primary reason identified by the audit for nitazoxanide use in the Top End of the NT between 2007 and 2012 was cryptosporidiosis. Cryptosporidium is a common cause of acute watery or persistent diarrhoea in immunocompetent and immunocompromised children, especially in developing countries21. Cryptosporidiosis is a notifiable disease. It is generally self-limiting in immunocompetent patients, although recurrent symptoms develop in 40% of cases following initial resolution, and 45% develop persistent diarrhoea (>14 days)22. There are no studies comparing Cryptosporidium infection or treatment with nitazoxanide in Indigenous versus non-Indigenous subjects. However, it is clear that infections within Indigenous communities are common, and are likely facilitated by the low infectious dose and prolonged shedding of the Cryptosporidium oocytes in the stools of infected patients (up to 5 weeks)21 .
During the study period, diagnosed Cryptosporidium diarrhoea accounted for 1.5% (n=105) of all infectious diarrhoea admissions. Only 27% of those with Cryptosporidium infection received treatment with nitazoxanide, mostly for persistent diarrhoea. Untreated patients were not assessed in this study; however, it is possible that failure to treat reflects a milder illness and/or a lack of awareness of potential nitazoxanide efficacy by the treating clinician. None of the treated children were found to have Giardia, amoebiasis, rotavirus or norovirus, against which nitazoxanide may also have efficacy23.
Whilst a number of trials of nitazoxanide have described beneficial effects, existing evidence is not sufficiently robust to support the routine use of nitazoxanide for infectious diarrhoea. The drug's manufacturer has sponsored all except one of the published trials24, and only small numbers have been randomised to the treatment intervention (n=176)4,9,13,24,25. In addition, all trials have been performed outside of Australia, and therefore the applicability of results to this study population remains uncertain. An effective therapy for infectious diarrhoea in the Northern Territory would clearly have significant community benefits. Improved resolution of diarrhoea could result in cost savings due to reduced number of hospital admissions, reduced length of stay, and decreased aeromedical retrieval costs.
Notably, whilst other antimicrobials for Cryptosporidium have been studied, such as macrolides and paramomycin, evidence for their efficacy is poor21, and they are not routinely used in practice.
Most children treated with nitazoxanide had resolution of diarrhoea in less than 3 days, consistent with the literature4. Resolution of diarrhoea is likely to have contributed to the weight gain observed in treated children. However, interpretation of this finding is confounded by both the enteral and intravenous rehydration and nutritional rehabilitation that was clinically prescribed for most children.
There was a large discrepancy between clinical estimates of dehydration versus those based on 'well weights', which suggested greater degrees of dehydration. The literature suggests that clinicians tend to over-estimate dehydration in children26. Persistent diarrhoea was present in the majority of cases in this study, and it is likely that 'well weight' estimates reflected, at least in part, weight loss due to persistent diarrhoea rather than acute dehydration per se.
Three-day dosing regimens recommended by the CDC for immunocompetent persons with Cryptosporidium diarrhoea are 100 mg BD (1-3 years), 200 mg BD (4-11 years) and 500 mg BD (adults). In the present study, the CDC dosing regimens for patients greater than 12 months were followed. The CDC does not support nitazoxanide use in children less than 12 months because of inadequate data in this age group. Overall, 24 children less than 12 months have been randomised to the treatment intervention in two studies examining nitazoxanide for rotavirus gastroenteritis23,24. The varied dosing schedules prescribed for children in the present cohort of treated infants less than 12 months reflects the paucity of data to direct use in this age category20.
The absence of adverse effects reported in this cohort is consistent with published randomised trials where few adverse events were identified in either treated or placebo groups4,13. Even though the study cohort included nine children <12 months, it was reassuring to note there were no adverse effects reported and a good clinical response was achieved.
Limitations of this study include the lack of control data in infected children not treated with nitazoxanide and the retrospective case series design. Some studies report Cryptosporidium infection to be self-limiting in immunocompetent children20,21, raising the possibility that the observed benefit among treated children may have occurred without treatment. However, time to discharge, weight gain and timely resolution of diarrhoea following treatment commencement all provide supportive evidence of a treatment effect in this cohort.
Based on current evidence, it is reasonable to consider nitazoxanide for children with persistent diarrhoea, even in cases where no enteropathogen is isolated. The data suggest a role for nitazoxanide in Indigenous children with Cryptosporidium infection who also suffer malnutrition. However, further independent, appropriately powered, multi-centre, double-blinded placebo-controlled studies across a range of settings are needed to assess the broader efficacy and safety of nitazoxanide as an empiric treatment for paediatric diarrhoea. In particular, the pharmacokinetics and safety profile of nitazoxanide among infants less than 12 months old need further investigation. The data obtained in this audit have been used to inform the design of a randomised control trial of nitazoxanide for paediatric infectious diarrhoea in the Northern Territory.
Acknowledgements
The authors are grateful to the pathology, pharmacy and coding departments at the RDH for their assistance with data collection, and to Linda Ward for her statistical support.
References
1. Global Health Observatory. Causes of child mortality by country, 2000-2010. (Online) 2012 . Available: http://www.who.int/gho/child_health/mortality/mortality_causes_text/en/index.html (Accessed 20 September 2012).
2. Shu Qin Li SG, d'Espaignet ET, Barbara Paterson B. From infancy to young adulthood health status in the Northern Territory. Northern Territory: Department of Health and Community Services, 2006.
3. Brewster D. Diarrhoeal disease. In CARPA Manual. 4th edn. Alice Springs: CARPA Publications, 2002.
4. Rossignol JF, Lopez-Chegne N, Julcamoro LM, Carrion ME, Bardin MC. Nitazoxanide for the empiric treatment of pediatric infectious diarrhea. Transactions of the Royal Society of Tropical Medicine and Hygiene 2012; 106(3): 167-173.
5. White A. Nitazoxanide: a new broad spectrum antiparasitic agent. Expert Review of Anti-infective Therapy 2004; 2(1): 43-45.
6. Fox LM, Saravolatz LD. Nitazoxanide: a new thiazolide antiparasitic agent. Clinical Infectious Diseases 2005; 40(8): 1173-1180.
7. Therapeutic Goods Administration. Orphan drugs: nitazoxanide. (Online) 2012. Available: http://www.tga.gov.au/industry/pm-orphan-drugs.htm (Accessed 17 December 2012).
8. Yeung J, Roseby R, Higgins S. A retrospective review of the use of nitazoxanide in the treatment of Cryptosporidium in children in Alice Springs Hospital. Retrospective audit. Poster presentation, 2009.
9. Rossignol JF, Kabil SM, El-Gohary Y, Younis AM. Nitazoxanide in the treatment of amoebiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007; 101(10): 1025-1031.
10. Rossignol JF, Kabil SM, el-Gohary Y, Younis AM. Effect of nitazoxanide in diarrhea and enteritis caused by Cryptosporidium species. Clinical Gastroenterology and Hepatology 2006; 4(3): 320-324.
11. Rossignol JF, El-Gohary YM. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Alimentary Pharmacology & Therapeutics 2006; 24(10): 1423-1430.
12. Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of nitazoxanide. Journal of Infectious Diseases 2001; 184(1): 103-106.
13. Rossignol JF, Abu-Zekry M, Hussein A, Santoro MG. Effect of nitazoxanide for treatment of severe rotavirus diarrhoea: randomised double-blind placebo-controlled trial. Lancet 2006; 368(9530): 124-129.
14. Rossignol J KS, El-Gohary Y, Younis A. Effect of nitazoxanide in diarrhoea and enteritis caused by Cryptosporidium species. Clinical Gatroenterology and Hepatology 2006; 4(3): 320-324.
15. Northern Territory Department of Health. Population data file 1971-2010. NT: Northern Territory Department of Health, 2012.
16. World Health Organisation. International statistical classification of diseases and related health problems. 10th revision (ICD-10) version for 2010. (Online). Available: http://apps.who.int/classifications/icd10/browse/2010/en#/I (Accessed 2 December 2013).
17. Department of Health and Ageing. National Immunisation Programme Schedule (as at May 2012) (Online) 2012. Available: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/E875BA5436C6DF9BCA2575BD001C80BF/$File/nip-schedule-card-may2012.pdf (Accessed 20 September 2012).
18. Australian Bureau of Statistics. ASGC Remoteness Classification: purpose and use. Census paper no. 03/01. 2003 (Online). Available: http://www.abs.gov.au/websitedbs/d3110122.nsf/0/f9c96fb635cce780ca256d420005dc02/$FILE/Remoteness_Paper_text_final.pdf (Accessed 11 November 2013).
19. de Onis M, Onyango AW, Borghi E, Garza C, Yang H. Comparison of the World Health Organization (WHO) Child Growth Standards and the National Center for Health Statistics/WHO international growth reference: implications for child health programmes. Public Health Nutrition 2006; 9(7): 942-947.
20. Centers for Disease Control and Prevention. Parasite - Cryptosporidium. (Online) 2010. Available: http://www.cdc.gov/parasites/crypto/health_professionals/tx.html (Accessed 21 December 2012).
21. Ochoa T, White A. Feigin and Cherry's textbook of paediatric infectious diseases. Volume 1. 6th edn. Philadelphia: Saunders Elsevier, 2009.
22. Fletcher J. Cryptosporidiosis. In: RJ Feigin, J Cherry, GJ Demmler-Harrison, SL Kaplan, WJ Steinbach, P Hotez, J Fletcher (eds). Feigin and Cherry's textbook of pediatric infectious diseases. Volume 1. 6th edn. Philadelphia: Saunders Elsevier, 2009; 2869-2880.
23. Siddiq DM, Koo HL, Adachi JA, Viola GM. Norovirus gastroenteritis successfully treated with nitazoxanide. Journal of Infection 2011; 63(5): 394-397.
24. Teran CG, Teran-Escalera CN, Villarroel P. Nitazoxanide vs. probiotics for the treatment of acute rotavirus diarrhea in children: a randomized, single-blind, controlled trial in Bolivian children. International Journal of Infectious Diseases 2009; 13(4): 518-523.
25. Amadi B, Mwiya M, Musuku J, Watuka A, Sianongo S, Ayoub A, et al. Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet 2002; 360(9343): 1375-1380.
26. Duggan C, Refat M, Hashem M, Wolff M, Fayad I, Santosham M. How valid are clinical signs of dehydration in infants? Journal of Pediatric Gastroenterology and Nutrition 1996; 22(1): 56-61.
