full article:
Introduction
The term sickle cell disease (SCD) includes a variety of pathological conditions resulting from the inheritance of the HbS gene either homozygously or as a compound heterozygote with other interacting abnormal haemoglobin genes1. In Jamaica, a developing country of predominantly West African origins, the incidence of homozygous S (HbSS) is 1:300 births; heterozygote C (HbSC) 1:500 births; sickle-β+ Thallasemia 1:3000 births; and sickle-β0 Thallasemia 1:7000 births1. The HbS gene is present in 10% and HbC in 3.5% of the Jamaican population1.
The natural history of SCD is highly variable with clinical manifestations ranging from acute life-threatening infections to chronic sequelae such as chronic neuropsychological and locomotor disability. Further, some of the heterogeneity in clinical outcomes is related to the SCD genotype as subjects with HbSC disease generally tend to have less severe disease compared with HbSS. However in assessing the seriousness of this disease, the emotional and social impacts are often underestimated. For example it has been reported that subjects with SCD experience increased psychological morbidity, such as depression and social isolation. Psychological complications in patients with SCD mainly result from the impact of pain and symptoms on their daily lives, and society's attitudes towards them2-4.
Over the last two decades, it has gradually been recognized that the mere assessment of the clinical and laboratory indicators of an individual's illness may not be adequate to define the extent of the effects of the illness on that individual. Health professionals are beginning to understand that the 'personal' burden of disease should not be measured simply by assessing physical indicators of illness such as cardiac function, the size of a tumor, the level of blood pressure, or other such factors. Psychosocial factors such as pain, apprehension, restricted mobility and other functional impairments, difficulty fulfilling personal and family responsibilities, financial burden, and diminished cognition must also be encompassed5 to fully comprehend what the illness means to an individual. There has been a gradual move to incorporate the patient's point of view in the full assessment of the disease experience. The area of research that has resulted from this recognition is termed 'quality of life'. It is a broad-ranging concept affected in a complex way by the person's physical health, psychological state, personal beliefs, social relationships and their relationship to salient features of their environment. Quality of life (QOL) refers to people's ability to function in the ordinary tasks of living. Quality of life analyses are particularly helpful for investigating the social, emotional and physical effects of treatments and disease processes on people's daily lives; analyzing the effects of disease or treatment from the patient's perspective; and determining the need for social, emotional and physical support during illness.
Previous studies in the UK and USA that have examined QOL in SCD have reported decreased QOL compared with the general population2,6. However, whether differences existed by genotype or by sociodemographic factors within the SCD group is unclear. Within Jamaica also, there exist marked sociodemographic differences that could impact on the QOL of subjects with SCD. For example, 50% of the total population of 2.695 million people (estimated in 2003) lives in urban areas. In 1998, 15.9% of the population was reported as living in poverty with 72% of the poor living in rural areas7. A greater proportion of people from the rural population in Jamaica reported having chronic illnesses, with an even smaller proportion having health insurance of any kind (7.6% in rural areas vs 25% in urban areas)8. Further, 23% of people from rural Jamaica who reported having a chronic illness were not actively engaged in seeking health care, because of affordability, compared with 9.4% from urban areas.
In this study we sought to test the null hypothesis that there was no difference in QOL between urban and rural dwelling subjects. A secondary objective was to assess whether there were differences in QOL by genotype.
Methods
Study population
This was designed as an observational, analytical study. Both public and private sector providers serve the healthcare system in Jamaica. Approximately 62% of ambulatory care is provided by the private sector9. The public sector healthcare system is decentralized into four regions and provides both in-patient and ambulatory care. Specialist hospitals provide 38.2% of total beds for in-patient services. Of those seeking health care, 57% go to private sector doctors, 38% use the public sector facilities and 5% use both9. The Sickle Cell Unit (SCU) of the University of the West Indies (UWI) operates Jamaica's only comprehensive SCD centre, located in Kingston the urban capital in the southeastern health region. All patients with any of the sickle cell diseases who wish to attend the SCU are registered and provided clinical care at the unit. The SCU provides outreach services to patients with SCD in two rural areas of the island, the south and northwest health regions.
The primary aim was to determine difference in total SF-36 score between urban and rural samples. A sample size of 85 per group would allow us at alpha 0.05 and power of 90% to detect one-half of a standard deviation difference between both groups. The 'urban' sample consisted of those participants aged 18 years and over who were recruited from the clinic at the UWI; the 'rural' sample consisted of participants, also aged 18 years and over, enrolled for the study at the outreach clinics. A convenience sample was obtained of all patients presenting during April-June 2005 for routine health maintenance visits. An exclusion criterion was the presence of any acute illness at the time of interview. All patients who were approached agreed to take part in the project.
Study instrument
The Short Form 36 v2 (SF-36) measure was administered by a single interviewer to all participants after they had signed an informed consent form. The SF-36 is one of the most commonly utilized measures of QOL10 and has been used in numerous studies with chronic illnesses. Principal components analysis has been performed on the SF-36 in two independent samples of Jamaicans with SCD, and has shown that three dimensions may underlie the SF-36 for both groups. These dimensions could be labeled 'physical health', 'mental health' and 'role limitations'. This solution accounted for 45.8% of the variability underlying the SF-36 in one sample and 54.6% of the variability in the other sample11.
Total SF-36 scores were used as a final measure of QOL in the two samples of patients with SCD. The SF-36 subscales: SF_PH (physical health), SF_RL (role limitations), and SF_MH (mental health), were calculated for the two samples. Data were also collected on participants' age, sex, genotype, marital status, highest level of education, employment status and occupation. The categories for the education level and occupation were matched to those that are utilized by the Jamaican Government-based surveys: 'The Economic and Social Survey'12 which is conducted by the Planning Institute of Jamaica and 'The Labour Force'13 conducted by the Statistical Institute of Jamaica (STATIN). The occupations were classified according to the 'Jamaica Standard Occupational Classification'14 which was published by STATIN in 1991.
The study was granted ethical approval by the University of the West Indies/University Hospital of the West Indies Ethics Committee. Strict confidentiality was guaranteed. All data were stored at computers at a secured location, with access provided only to the researchers involved in the study. If participants were to experience any discomfort during the data collection process, provisions were made to refer for appropriate follow up. However, this did not prove necessary.
Statistical approach
All data were initially captured into Epidata for Windows (The EpiData Association; Odense, Denmark). The Epidata program contained logical edits and consistency checks to ensure the accuracy of data. All questions were answered by the participants and there was no imputation of missing values required. Data were analyzed with Stata for Windows v 8.2 (StataCorp; College Station, TX, USA). Data were expressed as frequencies or means with standard deviations. Differences in proportions between groups were tested with the Chi-square statistic and while differences in means were tested with the t-test. To determine the association between QOL scores and sociodemographic variables, stepwise multiple linear regression analyses were performed with QOL score as the outcome, and rural-urban group and genotype being forced terms in the model, and sociodemographic variables as predictor variables. The categorical variables were coded using the 'dummy coding' scheme. The p-value for addition to the model was p <0.05 and for removal from model p >0.1. A p <0.05 was considered statistically significant.
Results
The urban sample consisted of 166 patients and the rural sample consisted of 90 patients (Table 1). There were no statistically significant differences in the socio-demographic variables examined between the two groups, although there was a trend for the urban sample to be older (mean age ± SD: urban vs rural, 35.8 ± 12.5 vs 32.8 ± 11.2 years, p = 0.059).
The unadjusted total SF-36 scores, as well as all subscale scores, were significantly greater in the rural group compared with the urban group (Table 2). A stepwise multiple linear regression model with total SF-36 score as the outcome, rural-urban group and genotype being forced terms in the model and sociodemographic variables as predictor variables was performed to determine differences between rural and urban groups, and between genotype adjusted for variations in sociodemographics. The result of this analysis showed that being employed and being employed in higher level occupations such as 'professionals' were significant predictors of higher total QOL score (Table 3). The rural sample had higher total QOL scores adjusting for age, employment and educational status. Additionally the SC genotype had significantly greater total QOL scores compared with the homozygous sickle cell disease (SS) genotype.
The Total SF-36 score was then disaggregated into its component subscales and the determinants of the subscale scores examined independently. For the 'physical health' subscale scores, being from the rural sample, younger age and being employed were associated with improved physical health (Table 4). Being employed in lower level occupations was associated with lower physical health scores.
The rural sample had significantly higher 'mental health' scores compared with urban sample. Additionally, being employed and being in higher level occupations was significantly associated with greater mental health scores (Table 5). Having the heterozygous SC disease was also positively associated with better mental health scores, this effect almost reaching significance (p 0.057).
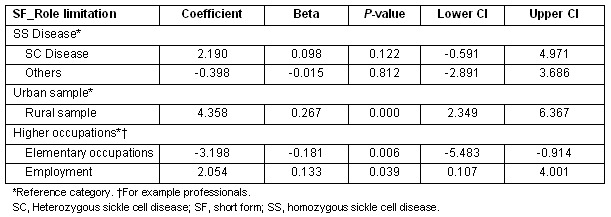
Patients who were employed, those in higher level occupations such as professionals, and those from the rural sample compared with urban, all showed lower limitations in their role functioning (Table 6).
Gender was not a significant predictor of either the total SF-score or any of the subscale scores.
Table 1: Sociodemographics of the two samples
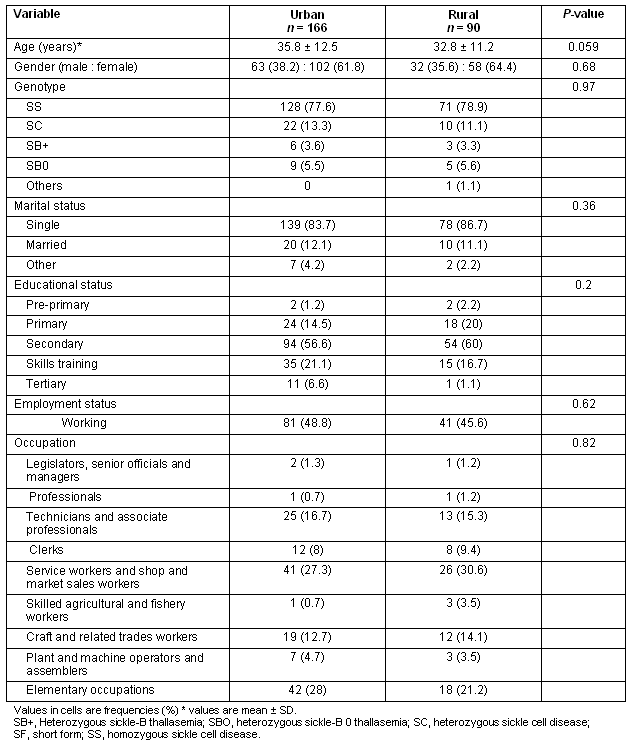
Table 2: Unadjusted total SF-36 scores and subscale scores by genotype and place of residence
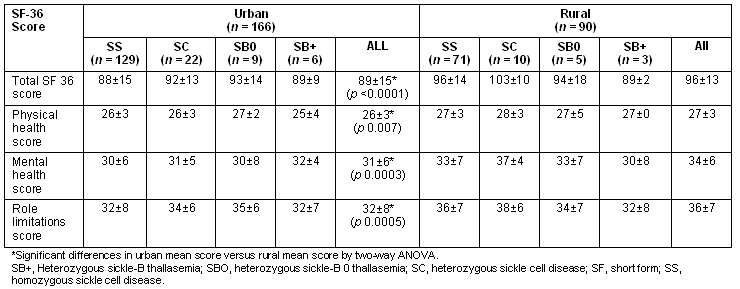
Table 3: Results of multiple regressions relating total SF score to exposure variables
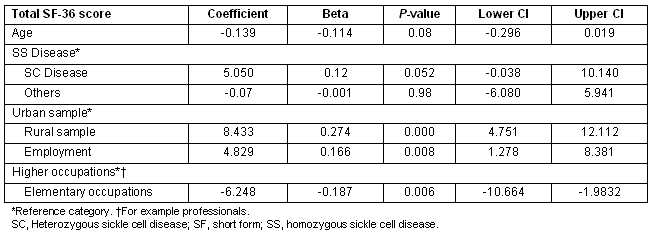
Table 4: Multiple regression showing predictors of SF_physical health scores
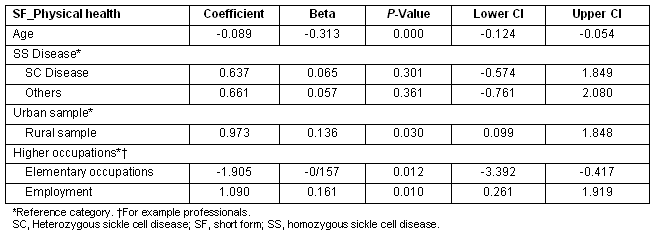
Table 5: Multiple regression showing Predictors of SF_mental health scores
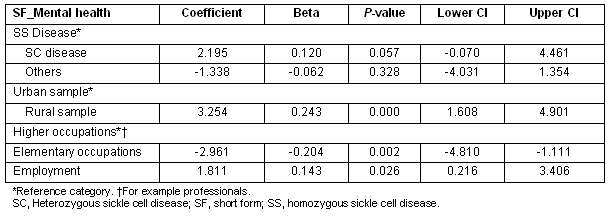
Table 6: Multiple regression showing predictors of SF_role limitation scores
Discussion
The aim of this study was to determine whether there were differences in QOL in an urban sample compared with a rural sample of subjects with SCD. The data reported here showed that total QOL score was significantly greater in the rural compared with the urban sample and SC genotype compared with SS genotype adjusting for sociodemographic variables of age, educational and employment status. Further, living in rural areas, being employed and being employed in more senior occupations were positive predictors for all three subscales of the SF-36. Increasing age was a significant negative predictor only for physical health but not for the other subscales.
These trends are to be expected because improved socioeconomic status (as measured by educational and employment levels in this study) as well as milder disease severity (as is usual with the heterozygous SC disease) would generally be associated with higher QOL. What was not anticipated, however, was that the 'rural' population with SCD exhibited higher total QOL scores than the 'urban' population.
Interestingly again, the rural patients have consistently shown significantly higher scores than the urban patients on the total SF-36 scale, as well as on all three subscales. This is despite the fact that there are no genotype differences between the samples, which is one of the indicators of severity of disease. The higher perceived QOL enjoyed by rural patients exists despite having far less access to health care. One possible explanation for these results concerns differences in levels of social support. In Jamaica, within the last two to three decades, there have been increasing migration rates from the rural to the urban areas, and the urban population in Jamaica has increased dramatically from 32% in 1960 to over 50% in 200012. As a result, these urban populations may not have the social and family support systems available to them anymore. This of course will also lead to greater challenges in managing their disease and, hence, perceptions of a poorer quality of life. Future research should investigate the role that differential levels of social support, coping styles, and perceptions of illness may play in urban-rural differences in QOL for SCD patients.
There are limited studies on rural/ urban differences in QOL in SCD. Telfair et al.15,16 have shown lower health service access and utilization in rural subgroups with SCD, as well as lower physical functioning for these rural groups. These results are also contrary to what has been shown in the present study. The only other study that has looked at SCD genotypes and their association with QOL6, has in fact reported better functioning on all subscales of the SF-36 in the more severe genotype group (ie SS/Sβ0 thal group) than in the less severe genotype group (ie SC/Sβ+ thal group). This is quite counter-intuitive and the present study has in fact shown the opposite, that is a better total QOL in the heterozygous SC disease patients than in those with SS disease.
Conclusions
This study has shown that rural populations with SCD in Jamaica enjoy better QOL than urban populations, on all dimensions of QOL. They also showed higher levels of mental health and functioning better despite limitations imposed on their physical health due to their chronic disease. One limitation of the current study is that a convenience sample rather than a random sample was studied. As such, the findings may not fully generalize to all patients with SCD. To better understand these surprising differences, future research using qualitative methods should be conducted to more fully elucidate the factors that lead rural patients in Jamaica to experience a higher QOL.
Acknowledgements
The authors would like to thank all the patients who so willingly participated in this study.
References
1. Serjeant GR, Serjeant BE. Sickle cell disease, 3rd edn. Oxford: Oxford University Press, 2001.
2. Anie KA. Psychological complications in sickle cell disease. British Journal of Haematology 2005; 129: 723-729.
3. Maxwell K, Streetly A, Bevan D. Experiences of hospital care and treatment seeking for pain from sickle cell disease: qualitative study. BMJ 1999; 318(7198): 1585-1590.
4. Midence K, Fuggle P, Davies SC. Psychosocial aspects of sickle cell disease (SCD) in childhood and adolescence: a review. British Journal of Clinical Psychology 1993; 32 (Pt 3): 271-280.
5. Muldoon MF, Barger SD, Flory JD, Manuck SB. What are quality of life measurements measuring? BMJ 1998; 316(7130): 542-545.
6. McClish DK, Penberthy LT, Bovbjerg VE, Roberts JD, Aisiku IP, Levenson JL et al. Health related quality of life in sickle cell patients: The PiSCES project. Health Quality and Life Outcomes2005; 3(1): 50.
7. PAHO. Health system and services profile of Jamaica. Washington, DC: Pan American Health Organization, 2001.
8. PIOJ, STATIN. Jamaica Survey of Living Conditions 2002. Kingston; December 2003.
9. Planning Institute of Jamaica. Economic and social survey of Jamaica, 2001. Kingston: PIOJ, 2002.
10. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992; 30: 473-483.
11. Asnani M, Lipps G, Reid M. Component structure of the SF-36 in Jamaicans with Sickle Cell Disease. West Indian Medical Journal2007; 56: 491-497.
12. Planning Institute of Jamaica. Economic and social survey Jamaica. Kingston: Planning Institute of Jamaica, 2003.
13. STATIN. The Labour Force. Kingston: The Statistical Institute of Jamaica, 2003.
14. STATIN. Jamaica standard occupational classification. Kingston: The Statistical Institute of Jamaica, 1991.
15. Telfair J, Haque A, Etienne M, Tang S, Strasser S. Rural/urban differences in access to and utilization of services among people in Alabama with sickle cell disease. Public Health Report 2003; 118: 27-36.
16. Haque A, Telfair J. Socioeconomic distress and health status: the urban-rural dichotomy of services utilization for people with sickle cell disorder in North Carolina. Journal of Rural Health 2000; 16: 43-55.


