full article:
Introduction
Breast cancer is the most common female cancer in the USA, making up approximately 26% of newly diagnosed cancers1. In 2008, an estimated 182 460 new cases of invasive breast cancer will be diagnosed and 40 480 deaths will result from this disease among US women1. If breast cancer is detected early, whether by screening mammography or breast examination, women have better treatment opportunities and improved survival1-4. The American Cancer Society (ACS) recommends an annual screening mammogram for women from age 40 years and older1; the National Cancer Institute (NCI) and the US Preventive Services Task Force recommend screening mammography every 1 to 2 years for the same age group5,6. In practice, 20-30% of women aged 50-64 years do not undergo mammography screening7, and even fewer adhere to the recommended intervals. Rakowski et al. found that among women aged 55-79 years, 51% had not returned for a repeat screening mammogram at the 12 month interval, and 36% had not returned within 24 months8. Clinical breast exams (CBE) and breast self exams (BSE) are recommended for women in their early 20s; however, research has shown that they play a small role in finding breast cancer9. For women at increased risk for breast cancer, for example due to a strong family history, the ACS recommends annual screening using magnetic resonance imaging (MRI) in addition to mammograms9.
Previous research indicates that many factors can influence a woman's likelihood of maintaining a regular schedule of mammogram screening for breast cancer: rural or urban residence10,11; convenience of accessing the mammogram including transportation12; distance from the nearest permanent and mobile mammography facility13; age14; race/ethnicity14; median county income11; years of education11; and confusion about the cost of the mammogram15. Stage at diagnosis has been associated with many factors including race16; obesity17; history of mammography17,18; method of tumor detection17; insurance status17,19; distance from residence to nearest hospital20; residence in rural areas21-23; residence in a nursing home24; and residence in areas of low socio-economic status17,25,26.
In a previous study, the present authors found that treatment choices among women with early stage breast cancer in the predominantly rural27 and seasonally cold28 state of New Hampshire were associated with travel distance to the nearest radiation treatment facility, and that women who chose lumpectomy were less likely to receive post-operative radiation therapy if diagnosed during winter months29. In the present study, it was hypothesized that geographic and seasonal factors affect the stage at which breast cancer is diagnosed. To determine if there are geographic barriers in cold, rural regions that may affect access to screening mammography, the present study assessed factors relating to accessibility, including travel time from patients' residences to the nearest mammography facility; rural or urban residence; season at time of diagnosis; and health insurance status were assessed. In addition, the effect of rural residence on stage at diagnosis was assessed.
Methods
Data
The study population was identified from the population-based New Hampshire State Cancer Registry (NHSCR). This statewide cancer surveillance program collects information on residents of New Hampshire diagnosed with and/or treated for in situ and invasive cancers by hospitals, physicians or other health care providers in the state. The registry also receives data for its residents who are diagnosed or treated elsewhere, primarily Massachusetts, Vermont, Maine, Florida, New York, and other states with cancer registries. Data quality and completeness meet the standards of the North American Association of Central Cancer Registries30.
The study population consisted of all female residents of New Hampshire with no prior history of cancer who were diagnosed between 1 January 1998 and 31 December 2004 with breast cancer histology codes 8500-8543 defined by ICD-O-2 for cases diagnosed from 1998-2000 and ICD-O-3 for cases diagnosed in years 2001-2004 (n=6305). Women diagnosed only at autopsy or identified only through death certificates were not included because of the unreliability of the diagnosis from this source. Because the American Cancer Society recommends annual mammography screening from age 401, women under this age were excluded (n=302).
The NHSCR collects stage at diagnosis classified according to the American Joint Commission on Cancer (AJCC) TNM Staging System. Each case in the dataset had either a pathologic or clinical AJCC stage group, which were pooled into combined stage groupings consisting of stages 0 (carcinoma in situ) to 4: If a patient had surgical excision with lymph node dissection, it meets the criteria for pathological staging. If surgery was not performed or if the surgery did not included a lymph node dissection, then stage is based on clinical findings. Where cases were staged both ways, pathological staging took precedence. When 37 women with unknown stage at diagnosis were excluded, 5966 patients remained. The rationale for including stages from 0 to 4 was that in situ and stage 1 cancers may be more likely to be identified through mammographic screening than by symptomatic disease31.
The registry collects demographic and clinical information, including patient residence, date of diagnosis, marital status, age, multiple primary cancers, tumor size, and insurance information when available. The season of diagnosis were classified as winter (December-February) or non-winter (March-November), as described previously29. Marital status at the time of diagnosis was defined as married or unmarried (single, separated, divorced, or widowed). Insurance status was classified as: (i) insured (health maintenance organizations, preferred provider organization, other managed care, TRICARE, Military, Veterans Administration, Indian and Public Health Service); (ii) Medicare, with and without supplemental insurance; (iii) Medicaid; (iv) not insured (which includes self-pay, charity write-off, and uninsured-not otherwise specified); and (v) unknown status.
Proximity of residence to nearest mammography facilities
All facilities certified to provide mammography services in New Hampshire (46) were identified, and the adjacent states of Maine (60), Massachusetts (181) and Vermont (26) during the years 1998-2004 from the US Food and Drug Administration (FDA), Center for Devices and Radiological Health32. Each facility's address was geocoded to the exact street location listed in the FDA file using ArcGIS 9.2 (Environmental Research Systems Institute [ESRI]; Redlands, CA). The addresses of patients were geocoded by TeleAtlas (Lebanon, NH ) to an exact street address (n=5457; 91.5%), or to the zip code geographic centroid if only a post office box or rural route address was provided (n=509; 8.5%). Using the Network Analyst extension in ArcGIS and data from ESRI on street networks and posted speed limits, driving distance and driving time from the patients' residence to the nearest mammography facility was calculated. It was not possible to account for variation in speed due to traffic congestion or weather. For analyses, the focus was on travel time rather than distance, to reflect the variation in commute times between urban and rural areas.
Urban/rural residence
Rural residence was considered as a secondary factor that might be associated with stage at diagnosis, while understanding the potential overlap between this variable and driving time to mammography facilities. To classify residence as rural or urban, the Rural Urban Commuting Area (RUCA) classification scheme was used. The RUCA system addresses some of the pitfalls of the county-based systems by creating categories at the US Census tract level and the ZIP code level. The classification system considers commuting patterns to larger metropolitan or large town area in designating categories within the system. The system has a total of 33 categories, which are commonly grouped together to form classifications of urban, large rural, and small rural33.
Access to primary care providers
Primary care supply was defined as the number of primary care (including obstetric and gynecology) providers (physicians, doctors of osteopathic medicine and physician assistants) in a given primary care service area (PCSA) per 1000 women aged 40 years and older34. Primary care supply was measured for each PCSA in New Hampshire (n=46, median size in km2 = 18.5, interquartile range [IQR] 8.85-44.26; in miles2 = 11.5, IQR 5.5-27.2) and assigned to the zip code tabulation area (ZCTA) of residence for individuals at the time of diagnosis. The PCSAs were developed by aggregating ZCTAs to reflect Medicare patient utilization of primary care providers34. They are based on 1999 and 2001 Medicare claims, 2000 US Census demographics, and 2000-2001 American Medical Association and American Osteopathic Association physician data. Nurse practitioner data are not included in these sources and were unavailable.
Statistical analysis
Descriptive analyses and tabulations for all variables was performed using chi-square analyses and 2-sided t-tests to compare predictor variables for each stage at diagnosis. Multivariate logistic regression models described factors that predict whether women were diagnosed with earlier stage (0-1) or later stage cancers (2-4). For multivariate logistic regression models, cases with unknown marital status were removed (n=133). The initial model included all categorical variables. However, travel time distribution showed an expected right-tailed skew; so this variable was log transformed before inclusion in subsequent models. This transformation had little effect on the results so travel time is also reported as a continuous variable. The Statistical Package for the Social Sciences (SPSS) v15 for Windows (SPSS Inc; Chicago, IL, USA; www.spss.com
Approval for the study of human subjects
This study was reviewed and approved by the Committee for the Protection of Human Subjects of Dartmouth College. Authorization was also granted by the State of New Hampshire, Department of Health and Human Services, Office of Community and Public Health, Bureau of Health Statistics and Data Management.
Results
The 5966 women included in the analysis had a mean age of 60.6 years (range 40-101). The distribution of cancer stages among all women was in situ (21.3%), 1 (41.9%), 2 (27.3%), 3 (6.6%) and 4 (2.9%). The mean driving distance between patient residence and the nearest mammography facility was 8.85 km (range 0-44.26, median 6.76, IQR 3.22-12.39; 5.5 miles, range 0-27.5, median 4.2, IQR 2-7.7), with a mean estimated travel time of 8.9 min (range 0-42.2, median 7; IQR 3.4-7). The distributions of these two variables were substantially skewed to the right: 56.3% of patients lived within 8 km (5 miles) of a mammography facility, and 65% had a travel time of less than 10 min. Driving distance to the nearest mammography facility was significantly correlated with driving time (r=.966; p=<0.001), which is expected, particularly in the more urban areas of the state where the majority of cases were located (urban 59.1%, rural 40.9%).
In univariate analyses, associations were seen between later stage (stage 2-4) diagnosis and either Medicaid coverage (p<0.0001) or lack of insurance (p<0.0001) (Table 1). Women aged 80 years or older were more likely to be diagnosed at a later stage (p<0.001), as were women who were not married (separated, divorced, or widowed) (p<0.001). No significant difference were found between women diagnosed with earlier or later stage breast cancer in the estimated driving time or distance to the nearest mammography facility, in the primary care provider density, season of diagnoses, or urban/rural residence.
Multivariate analyses confirmed that stage at diagnosis was associated significantly with health insurance status (p< 0.001), marital status (p<0.001) or age (p<0.001) (Table 2). Season of diagnosis contributed with borderline significance to the final multivariate model (p=0.074). Compared with women with private health insurance, the adjusted odds ratio for later stage at diagnosis among women using Medicaid was 2.01 (95% CI 1.27-3.16). No significant association was found between cancer stage at diagnosis and driving time to the nearest mammography facility either as a continuous or categorical variable. Primary care provider density and urban/rural residence were not significantly associated with stage at diagnosis (data not shown).
Table 1: Characteristics of cancer stage at diagnosis in 5966 New Hampshire women with breast cancer, 1998-2004
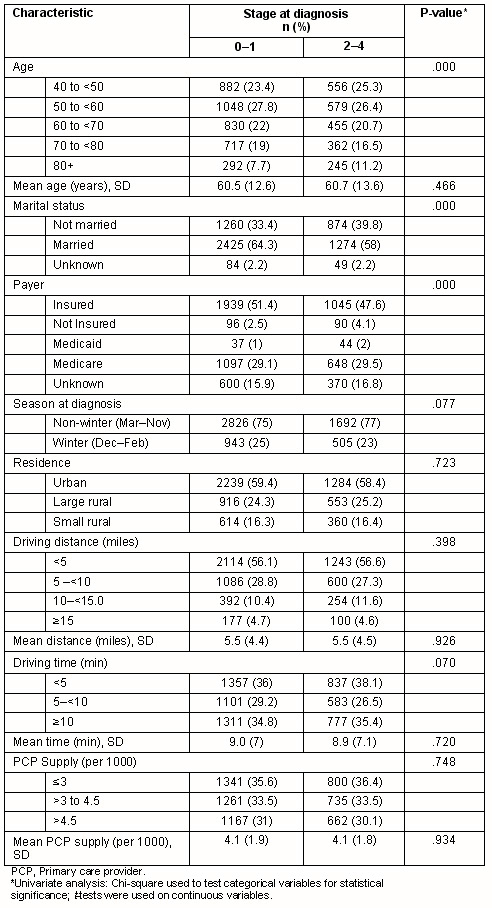
Table 2: Logistic regression models predicting later stage (stage 2-4) breast cancer at diagnosis among 5833 New Hampshire women diagnosed with breast cancer, 1998-2004?
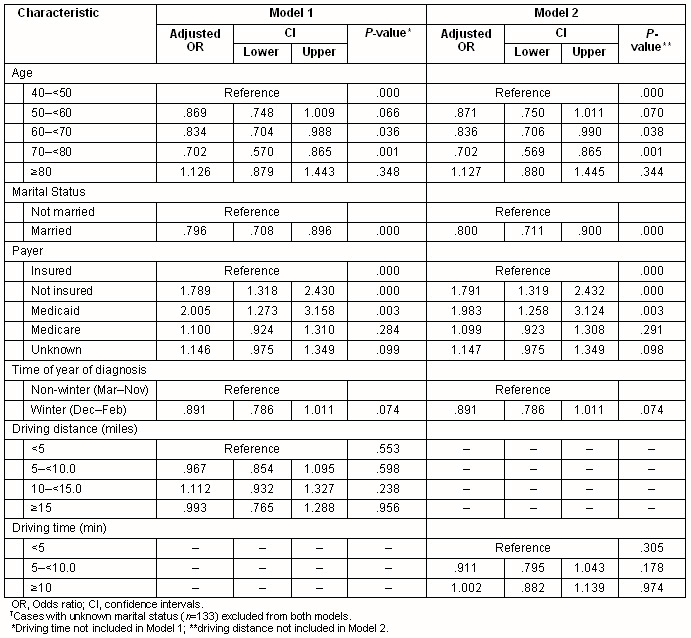
Interaction terms representing travel time and age, and travel time and health insurance type were not statistically significant. Further, in analyses restricted to women aged less than 65 years, health insurance status was a significant predictor of stage at diagnosis (p<0.001) but age, marital status and season of diagnosis were not. Among women aged 65 years or older, age and marital status were significantly associated with stage, but health insurance status (almost uniformly Medicare) was not (data not shown). Travel time was not significant in either of the models restricted by age. Analyses stratified on age group (above or below 75 years), or on rural residence, failed to identify any significant association between travel distance (or time) and the stage at diagnosis (data not shown).
To assess the most extreme stages at which breast cancer may be diagnosed, women with in situ cancer (stage 0) were also compared with those diagnosed at stages 3 or 4. The model showed a significant influence of health insurance (p=0.015), marital status (p=0.001) and age (p<.001) but again travel distance to a mammography facility was not statistically significant (data not shown). Similar results were obtained in comparing stages 0-2 with 3-4.
Discussion
The data indicate that in the predominantly rural state of New Hampshire, mammography facilities are geographically accessible to most women, and that neither travel distance/time to mammography nor rural residence significantly delays the diagnosis of breast cancer. Other, non-geographic factors present more substantial risks for a late stage diagnosis of breast cancer, with higher risk among the uninsured, elderly and those who are not married. Prior studies support the observation that having no health insurance or being covered by Medicaid was significantly associated with a later stage at diagnosis when compared with having private health insurance17,35,36. The results are reassuring because they suggest that rural residence in New Hampshire does not necessarily present a significant disadvantage to women in relation to breast cancer diagnosis, and their geographic access to mammography is generally good.
Recent studies along the same lines have produced inconsistent results. A study in Kentucky reported a significant association between later stage at diagnosis and increasing travel distance to mammography37, and a Texan study reported a significantly increased risk of late stage breast cancer diagnosis among residents of numerous counties that lacked any mammography facility38. In contrast, reports from Virginia39 and Illinois40, like the present study, found no association between travel distance to mammography and later stage diagnosis of breast cancer. The mean travel distance to mammography (9.65 km; 6 miles) in the Kentucky report was similar to that seen in New Hampshire (8 km; 5 miles). In Kentucky, an excess of late stage (III and IV) cancers was found among those living 24.14 km (15 miles) or more from a mammography facility; 12.7% compared to only 8.7% of (0-II) stage cancers. The corresponding proportions in New Hampshire were 5.1% and 4.6%, suggesting that even though twice as many women were analyzed in Kentucky as in New Hampshire, the discrepancy in these results was not necessarily attributable simply to sample size. The reasons for the apparently contradictory results from these studies are unclear, but it seems likely that states vary not only in terms of geographic and other factors affecting stage at diagnosis, but also in researchers' ability to collect and analyze data that adequately represent those factors.
The Illinois study of Medicare recipients40, as well as a British study41, while reporting that geographic access to mammography facilities was not an important factor in the diagnosis of late-stage cancer, found that the availability of primary care facilities was significant. No evidence was found that the density of primary care providers in the PCSA for each woman's residence affected the stage at which breast cancer was diagnosed. The use of per capita primary care provider (PCP) supply as a proxy for the availability of primary care to the individual has potential drawbacks, as it is a group-level measure that may differ among individuals within primary care service areas42. The present study's exclusion of nurse practitioners (NP) from the PCP supply measure is unlikely to introduce bias since most NPs practice in the same physical location as other providers, and thus would be unlikely to contribute an independent practice location to the supply measure.
Other studies which categorized women's residences as urban or rural, found that women residing in rural areas are diagnosed with breast cancer at a later stage25. Colorectal cancer patients living in rural areas were found to travel long distances for cancer treatment43. Paquette and Finlayson found that urban rather than rural populations were associated with later stage at diagnosis for lung and colorectal cancer, but their designation of rural versus urban has limitations44. Defining urban and rural populations in New Hampshire is not necessarily straightforward. In New Hampshire, 84% of the state's land mass is rural, and this same area accounts for 37% of the population27. Whereas many cities elsewhere show distinct segregation of rich and poor, employed and unemployed in specific neighborhoods, the economic and social characteristics of the inhabitants of a given census tract in New Hampshire are highly variable. Thus, group level ecological measures in the New Hampshire population may be imprecise markers for the individuals they are supposed to represent.
It was originally hypothesized that the season in which breast cancer is diagnosed may be associated with stage because severe winter weather conditions may deter women from traveling for mammography. After adjustment for other factors, it was found that a small excess of late stage cancers (77% versus 75%) were diagnosed during non-winter seasons (p=0.074). In the authors' previous study, it was found that women diagnosed during winter were more likely to forego recommended treatment29. Researchers from Maine, a larger, neighboring state with similar geographic characteristics but a more sparse population45,46, used climatological data to investigate weather as a predictor of late stage prostatic and colorectal cancers, with negative results. The timing and pattern of use of mammography facilities by individuals before a diagnosis of cancer could be investigated in future studies.
The strengths of the study include the use of population-based central cancer registry data, noted for achieving 99% case ascertainment and high quality data47. Unlike similar studies in which travel time was based on zip code areas13,40, the estimations of driving distance and travel time were derived in most cases from the exact street address of a patient's primary residence at diagnosis to the exact location of the nearest mammography facility, although the estimates may not reflect road quality particularly during snowy and icy days or traffic congestion.
The limitations of this study include a lack of knowledge about the actual use of mammography facilities for screening, diagnostic work-up of clinical disease, or indeed whether the nearest facility was used by a given patient. Tumors detected during screening tend to be less aggressive than those presenting with symptoms between regularly-scheduled mammograms ('length biased sampling'). Thus, a proportion of later stage tumors arise among women who follow standard screening recommendations, and these women cannot be identified from the data. A screening history for each woman might have helped elucidate the role of geographic barriers among individual women in the study. Previous research has shown that women with late stage breast cancer were half as likely to have had a screening mammogram within the previous 13-36 months than those with early stage disease, particularly if they were older than 75 years of age, unmarried, or without a family history of breast cancer48. Residence in census blocks with less education or lower median income is also associated with lack of screening48. These factors may have impacted stage at diagnosis in the study as well as other factors which could not be accounted for, including availability of public transportation or a personal vehicle and the relative costs of mammography at different facilities under different insurance plans.
Although predominantly rural, New Hampshire is a small state that seems to be well served with mammography facilities; this may not be true of other, rural states whose populations must travel greater distances to access health care. The fact that the findings differ from other studies of rural areas suggests that populations which would conventionally be classified as rural may be heterogeneous in terms of important health predictors such as geographic access to care, insurance status and social support networks. A multi-state collaboration addressing these issues would assist understanding of the similarities and differences in these other factors among rural states in relation to cancer prevention.
Conclusion
Breast cancer stage at diagnosis in New Hampshire does not seem to be attributable to geographic barriers to mammography screening facilities, but is significantly associated with health insurance, marital status, and age at diagnosis. It seems likely that, while gaps in mammographic screening are not generally related to geographic barriers, a complex variety of other factors may affect a woman's motivation or ability to screen, including financial and social incentives.
Acknowledgements
The authors thank Dr Karla R Armenti and Dr Jose Montero who oversee the State Cancer Registry at New Hampshire Department of Health and Human Services, and Ms Mary Lewis who oversees the Registry on behalf of the National Program of Cancer Registries. NHSCR is supported by the Centers for Disease Control and Prevention's National Program of Cancer Registries (NPCR) through cooperative agreement U58/DP000798 awarded to the New Hampshire Department of Health and Human Services, Division of Public Health Services, Bureau of Disease Control and Health Statistics, Health Statistics and Data Management Section. The contents of this article are solely the responsibility of NHSCR and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
References
1. American Cancer Society. Cancer facts & figures 2008. Atlanta: American Cancer Society; 2008.
2. Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M et al. Effect of screening and adjuvant therapy on mortality from breast cancer. New England Journal of Medicine 2005; 353: 1784-1792.
3. Lee CH. Screening mammography: proven benefit, continued controversy. Radiologic Clinics of North America 2002; 40(3): 395-407.
4. Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomized trials. Lancet 2002; 359(9310): 909-919.
5. National Cancer Institute. Screening mammograms: questions and answers. Fact sheet 5.28. (Online) no date. Available: http://www.cancer.gov/cancertopics/factsheet/Detection/screening-mammograms (Accessed 29 May 2008).
6. US Preventive Services Task Force. Screening for breast cancer: recommendations and rationale. (Online) 2009. Available: http://www.ahrq.gov/clinic/uspstf/uspsbrca.htm (Accessed 29 May 2008).
7. Ostbye T, Greenberg GN, Taylor DH Jr, Lee AM. Screening mammography and Pap tests among older American women 1996-2000: results from the Health and Retirement Study (HRS) and Asset and Health Dynamics Among the Oldest Old (AHEAD). Annals of Family Medicine 2003; 1(4): 209-217.
8. Rakowski W, Breen N, Meissner H, Rimer BK, Vernon SW, Clark MA et al. Prevalence and correlates of repeat mammography among women aged 55-79 in the year 2000 National Health Interview Survey. Preventive Medicine 2004; 39(1): 1-10.
9. American Cancer Society. Cancer prevention & early detection facts & figures 2009. Atlanta, GA: American Cancer Society, 2009.
10. Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Social Science & Medicine 2008; 66(2): 260-275.
11. Calle EE, Flanders WD, Thun MJ, Martin LM. Demographic predictors of mammography and Pap smear screening in US women. American Journal of Public Health 1993; 83(1): 53-60.
12. Zenk SN, Tarlov E, Sun J. Spatial equity in facilities providing low- or no-fee screening mammography in Chicago neighborhoods. Journal of Urban Health: Bulletin of the New York Academy of Medicine 2006; 83(2): 195-210.
13. Engelman KK, Hawley DB, Gazaway R, Mosier MC, Ahluwalia JS, Ellerbeck EF. Impact of geographic barriers on the utilization of mammograms by older rural women. Journal of the American Geriatrics Society 2002; 50(1): 62-68.
14. Edwards QT, Li AX, Pike MC, Kolonel LN, Ursin G, Henderson BE et al. Ethnic differences in the use of regular mammography: the multiethnic cohort. Breast Cancer Research and Treatment 2009; 115(1): 163-170.
15. McAlearney AS, Reeves KW, Tatum C, Paskett ED. Perceptions of insurance coverage for screening mammography among women in need of screening. Cancer 2005; 103(12): 2473-2480.
16. Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL et al. Trends in breast cancer by race and ethnicity: update 2006. CA: A Cancer Journal for Clinicians 2006; 56(3): 168-183.
17. Hahn KME, Bondy ML, Selvan M, Lund MJ, Liff JM, Flagg EW et al. Factors associated with advanced disease stage at diagnosis in a population-based study of patients with newly diagnosed breast cancer. American Journal of Epidemiology 2007; 166(9): 1035-1044.
18. Coates RJ, Uhler RJ, Brogan DJ, Gammon MD, Malone KE, Swanson CA et al. Patterns and predictors of the breast cancer detection methods in women under 45 years of age (United States). Cancer Causes and Control 2001; 12(5): 431-442.
19. Pollitt RA, Clarke CA, Shema SJ, Swetter SM. California medicaid enrollment and melanoma stage at diagnosis: a population-based study. American Journal of Preventive Medicine 2008; 35(1): 7-13.
20. Silverstein MD, Nietert PJ, Ye X, Lackland DT. Access to care and stage at diagnosis for patients with lung cancer and esophageal cancer: analysis of the Savannah River Region Information System cancer registry data. The Southern Medical Journal 2002; 95(8): 900-908.
21. Amey CH, Miller MK, Albrecht SL. The role of race and residence in determining stage at diagnosis of breast cancer. Journal of Rural Health 1997; 13(2): 99-108.
22. Liff JM, Chow WH, Greenberg RS. Rural-urban differences in stage at diagnosis. Possible relationship to cancer screening. Cancer 1991; 67(5): 1454-1459.
23. Campbell NC, Elliott AM, Sharp L, Ritchie LD, Cassidy J, Little J. Rural and urban differences in stage at diagnosis of colorectal and lung cancers. Cancer 2001; 84(7): 910-914.
24. Bradley CJ, Clement JP, Lin C. Absence of cancer diagnosis and treatment in elderly Medicaid-insured nursing home residents. Journal of the National Cancer Institute 2008; 100(1): 21-31.
25. MacKinnon JA, Duncan RC, Huang Y, Lee DJ, Fleming LE, Voti L et al. Detecting an association between socioeconomic status and late stage breast cancer using spatial analysis and area-based measures. Cancer Epidemiology Biomarkers and Prevention 2007; 16(4): 756-762.
26. Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer 2006; 107(5 Suppl): 1189-1195.
27. New Hampshire Department of Health and Human Services, Division of Public Health Services. NH Rural Health Report. Concord, NH: New Hampshire Department of Health and Human Services, 2004.
28. State of New Hampshire. The New Hampshire almanac. Fast New Hampshire facts. (Online) no date. Available: http://www.nh.gov/nhinfo/fastfact.html (Accessed 29 May 2008).
29. Celaya MO, Rees JR, Gibson JJ, Riddle BL, Greenberg ER. Travel distance and season of diagnosis affect treatment choices for women with early-stage breast cancer in a predominantly rural population (United States). Cancer Causes and Control 2006; 17(6): 851-856.
30. Havener L (Ed.). Standards for cancer registries Volume III: Standards for completeness, quality, analysis, and management of data. Springfield, IL: North American Association of Central Cancer Registries, 2004; 48.
31. Claus EB, Stowe M, Carter D. Breast carcinoma in situ: risk factors and screening patterns. Journal of the National Cancer Institute 2001; 93(23): 1811-1817.
32. US Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health. Mammography facilities. (Online) no date. Available: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMQSA/mqsa.cfm (13 June 2007).
33. University of Washington, WWAMI Rural Health Research Center. Using RUCA data. (Online) no date. Available: http://depts.washington.edu/uwruca/ruca-codes.php (Accessed 14 April 2008).
34. Goodman DC, Mick SS, Bott D, Stukel T, Chang CH, Marth N et al. Primary care service areas: a new tool for the evaluation of primary care services. Health Services Research 2003; 38(1Pt 1): 287-309.
35. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. The Lancet Oncology 2008; 9(3): 222-231.
36. Roetzheim RG, Pal N, Tennant C, Voti L, Ayanian JZ, Schwabe A et al. Effects of health insurance and race on early detection of cancer. Journal of the National Cancer Institute 1999; 91(16): 1409-1415.
37. Huang B, Dignan M, Han D, Johnson O. Does distance matter? Distance to mammography facilities and stage at diagnosis of breast cancer in Kentucky. Journal of Rural Health 2009; 25(4): 366-371.
38. Elting LS, Cooksley CD, Bekele BN, Giordano SH, Shih YC, Lovell KK et al. Mammography capacity impact on screening rates and breast cancer stage at diagnosis. American Journal of Preventive Medicine 2009; 37(2): 102-108.
39. Schroen AT, Lohr ME. Travel distance to mammography and the early detection of breast cancer. The Breast Journal 2009; 15(2): 216-217.
40. Wang F, McLafferty S, Escamilla V, Luo L. Late-stage breast cancer diagnosis and health care access in Illinois. The Professional Geographer 2008; 60(1): 54-69.
41. Jones AP, Haynes R, Sauerzapf V, Crawford SM, Zhao H, Forman D. Travel times to health care and survival from cancers in Northern England. European Journal of Cancer 2008; 44(2): 269-274.
42. Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the US. Cancer 2008; 112(4): 909-918.
43. Baldwin LM, Cai Y, Larson EH, Dobie SA, Wright GE, Goodman DC et al. Access to cancer services for rural colorectal cancer patients. Journal of Rural Health 2008; 24(4): 390-399.
44. Paquette I, Finlayson SR. Rural versus urban colorectal and lung cancer patients: differences in stage at presentation. Journal of the American College of Surgeons 2007; 205(5): 636-641.
45. Parsons MA, Askland KD. Determinants of prostate cancer stage in northern New England: USA Franco-American contextual effects. Social Science & Medicine 2007; 65(10): 2018-2030.
46. Parsons MA, Askland KD. Cancer of the colorectum in Maine, 1995-1998: determinants of stage at diagnosis in a rural state. Journal of Rural Health 2007; 23(1): 25-32.
47. National Program of Cancer Registries. Data completeness and quality audits, New Hampshire State Cancer Registry, diagnosis year: 2004. Concord, NH: NPC, 0000.
48. Taplin SH, Ichikawa L, Ulcickas Yood M, Manos MM, Geiger AM, Weinmann S et al. Reason for late-stage breast cancer: absence of screening or detection, or breakdown in follow-up? Journal of the National Cancer Institute 2004; 96(20): 1518-1527.

