Miwatj Health is an Aboriginal community controlled health service situated in East Arnhemland. In September 2001, after extensive consultation with Aboriginal people in the area, Miwatj Health together with the School of Public Health and Tropical Medicine, James Cook University, Townsville sponsored the 'First National Workshop on Strongyloidiasis' in Nhulunbuy, Northern Territory, Australia. The aim of the workshop was to set a platform to educate the Aboriginal people of East Arnhem Land about the existence of strongyloidiasis, to highlight methods of eliminating the disease and to give them the option of deciding how to address the issue. Organisers of the workshop also invited health professionals and clinicians who work with Aboriginal communities, and experts with an interest in parasitology to participate by sharing their interest and expertise through the exchange of information and ideas. During the workshop, a senior medical officer employed by Miwatj Health stated her belief that strongyloidiasis was a significant health issue in her community, and expressed frustration at the apparent dismissal of strongyloidiasis as a public health issue by health authorities. The workshop was dedicated to a senior Aboriginal health worker who had died from disseminated strongyloidiasis and had dedicated her life to improving the health of her people. A senior Indigenous participant asked, 'We've had this strongyloides for so long, why hasn't something been done about it?'
An examination of unpublished literature and a collection of anecdotal case reports, suggests that strongyloidiasis is a major problem in some rural and remote Aboriginal communities, causing minor disease in many people and occasionally resulting in severe illness and even death (W Page, pers. obs, 2001)1-3.
Transmission of Strongyloides stercoralis, the causative organism, does not occur in the urban Australian environment, and cases are found only among people who have acquired the parasite in countries where it is endemic. Doctors and nurses trained in urban settings are usually unaware of strongyloidiasis, and although the consequences of infection can be fatal, they may fail to consider it in their differential diagnoses4. In remote Aboriginal communities in northern Australia where Strongyloides is hyperendemic2,5-9 failure to make the diagnosis has a great impact on preventable morbidity and mortality due to delayed presentation and reduced access to clinical and tertiary care4. Hence, there is an urgent need for medical and nurse clinicians working with remote Aboriginal communities to be fully aware of Strongyloides and its significance.
Strongyloidiasis
The highest prevalence in the world of Strongyloidiasis, a disease caused by the parasitic gut nematode (roundworm), Strongyloides stercoralis, is in the rural and remote Aboriginal communities of northern Australia2. With prevalences of greater than 25%, these communities have rates of strongyloidiasis higher than those in the worst affected developing countries where surveys have been recently conducted2,4,5. In one remote Aboriginal community, 41% of those tested were positive on a single stool specimen2.
Life Cycle
The key to breaking transmission of Strongyloides is stopping infective larvae from penetrating the skin. If faeces are indiscriminately deposited on soil, the larvae of Strongyloides grow to become infective larvae within 3 days. These are very much like hookworm larvae and enter the human body by penetrating intact skin.
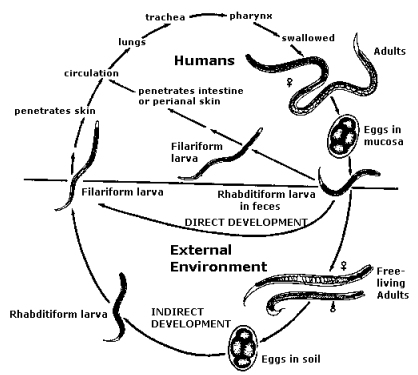
Figure 1: Life cycle of Strongyloides stercoralis
The unique feature of the life cycle of Strongyloides stercoralis (Figure 1) is that infective larvae form inside the human body. These are called autoinfective larvae. The autoinfective larvae have three main effects:
- The autoinfective larvae make the infection life-long and patients with strongyloidiasis are infected for life unless proven otherwise10 because adult worms re-invade the small intestine even without new infective larvae invading from the external environment.
- The autoinfective larvae may lead to a massive build up in numbers of worms. This terminal state of strongyloidiasis is called hyperinfection and happens particularly when immunosuppressive drugs are used.
- Because the autoinfective larvae invade back into the body mainly from the large intestinal lumen, they carry enteric bacteria to many organs. Recurrent sepsis with enteric bacteria is a hallmark of a case of strongyloidiasis that is becoming severe. Few primary health-care personnel think of strongyloidiasis when their Aboriginal patient presents with septicaemia or even pneumonia. A sad and sometimes fatal oversight!4
Clinical presentation of strongyloidiasis
Strongyloidiasis has three main presentations: acute, chronic uncomplicated and disseminated.
Acute strongyloidiasis usually seen in children and is manifested by diarrhoea, low plasma protein (hypoproteinemia) and low serum potassium (hypokalaemia)6.
Chronic uncomplicated strongyloidiasis is manifested by epigastric pain, recurrent hives (urticaria) and transient red lines on the skin that appear and move rapidly (larva currens) and episodic diarrhoea11.
Disseminated strongyloidiasis ('hyperinfective syndrome' or hyperinfection) has a range of manifestations but often these are sepsis, respiratory and gut signs with a high case fatality rate11. Patients who develop the hyperinfective syndrome are usually immunocompromised, immunodepressed or malnourished, although a small number of case reports have no obvious predisposing condition.
Other clinical manifestations include paralytic ileus, severe diarrhoea, pneumonia, pulmonary haemorrhage, septicaemia or other bacterial infections.
The diagnosis of strongyloidiasis is usually made by serological test to detect antibodies against Strongyloides in the blood, or by finding larvae in faeces. The serological tests are performed at specialised laboratories in Sydney, Perth or Brisbane. Strongyloidiasis, whether chronic uncomplicated or the hyperinfective syndrome, is rarely diagnosed unless the clinician and laboratory are specifically looking for the disease.
Despite corticosteroids and immunosuppressants precipitating fatal disseminated strongyloidiasis, there is no mention of this in warnings in drug literature in Australia.
Epidemiology of strongyloidiasis
The prevalence of strongyloidiasis in Aboriginal community is much higher than in mainstream communities, and cases of strongyloidiasis acquired by Caucasians in Australia are rare compared with those among Aboriginal people.
Globally, any community with a prevalence of Strongyloides greater than 5% is classed as hyperendemic11, the most severe epidemiological category. Many Aboriginal communities in Northern Australia fall into the hyperendemic category.
In the Australian population, high-risk groups are:
- Aboriginal people living in remote communities in the Northern Australia Some communities have estimates of up to 60% prevalence3,5 .
- Immigrants from Southeast Asia, particularly Vietnam, Cambodia and Laos;
- Prisoners of War (POWs) from Southeast Asia in WWII;
- Military who served in Southeast Asia (e.g. Vietnam veterans);
- Any patient with recurrent urticaria, larva currens or eosinophilia.
It appears that there are insufficient data available to convince health authorities that strongyloidiasis is a major concern in Aboriginal communities. In part, this is because data collected in surveys of individual communities have not been published. Because the data have not been collected systematically and strongyloidiasis is not an overt disease, the full extent of the problem is as yet unquantified and so has a low profile among health departments and clinicians.
The available literature of published case reports (mostly from outside Australia) extends from 1876 to 2002 and shows that most cases of death from hyperinfection are usually due to:
- Missed diagnosis: no diagnostic tests performed until after the patient's death or too late in the disease for the treatment to be effective.
- Patient immunosuppression combined with the patient not being checked for Strongyloides .
- Patient diagnosed with Strongyloides and treated but not followed up to confirm a cure. When this happens, some patients represent with hyperinfection a year or more after the first episode.
Treatment
The two oral drugs used in therapy for strongyloidiasis are ivermectin and albendazole. The weight of published evidence favours ivermectin as the most effective treatment12-14. Doses for adults are 0.2 mg/kg ivermectin as a single dose, or 400 mg albendazole per day for 3 days. Because the autoinfective larvae are difficult to eradicate, one dose may be insufficient and re-treatment of patients on two occasions, at 1 and 2 months after the initial treatment dose is recommended by the authors. This re-treatment regimen has yet to be proven in clinical trials, and could be criticised as excessive, however the seriousness of the infection warrants aggressive treatment. In Australia, an effective treatment is available because both ivermectin and albendazole are registered for strongyloidiasis treatment.
It is worthwhile noting that one of the world's leading medical books states, 'Even in the asymptomatic state, strongyloidiasis must be treated because of the potential for fatal hyperinfection'15.
Summary
Strongyloidiasis is a disease for which effective and safe treatments are available. Most patients die from strongyloidiasis because the disease is not diagnosed and goes untreated, or the disease is diagnosed and partly treated but management is inadequate, or because immunosuppressant drugs and regimens are commenced in patients from high-risk groups without strongyloidiasis being tested for and diagnosed.
If not adequately treated, strongyloidiasis can cause serious illness with a high case fatality rate due to infection, or alimentary or pulmonary complications. The treatment of strongyloidiasis in Indigenous patients is unsatisfactory because repeat courses of treatment are rarely given, the follow-up process is often incomplete and there is no patient register to monitor cure of strongyloidiasis.
Conclusion
The only way to deal with the strongyloidiasis problem in Australian Indigenous communities is for strongyloidiasis to be recognised and addressed at the national level. In urgent need of action is the formulating and costing of a detailed plan for intervention, which includes health education, public health interventions (such as instructions about establishing pit laterines) and guidelines for mass treatment. Finally, Health Departments must accept responsibility for establishing appropriate systems of effective treatment for, and monitoring of individuals with strongyloidiasis.
References
1. Byard RW, Bourne AJ, Matthews N et al. Pulmonary strongyloidiasis in a child diagnosed on open lung biopsy. Surgical Pathology. 1993; 5: 55-62.
2. Hansman D. Public Health Information. A rapidly progressive fatal illness associated with strongyloidiasis. Communicable Disease Report. Adelaide: Women's and Children's Hospital. 1995.
3. Flannery G, White N. Immunological parameters in northeast Arnhem Land Aborigines: consequences of changing settlement and lifestyles. In: LM Schell, MT Smith, A Bilsborough (Eds). Urban Ecology and Health in the Third World. Cambridge UK: Cambridge University Press, 1993; 202-220.
4. Speare R, White S. Strongyloidiasis - a social determinant of health? Outback Flyer 2001; 50: 4-5.
5. Aland K. Worm Project at Galiwin'ku. Working Together. 1996; 6: 10.
6. Kukuruzovic R, Brewster D. Diarrhoeal disease in top end Aboriginal children. Occasional Papers Issue 1. Darwin: Flinders University, Northern Territory Clinical School, 2002.
7. Fisher D, McCarry F, Currie B. Strongyloidiasis in the Northern Territory. Under-recognised and under-treated? Medical Journal of Australia 1993; 159: 88-90.
8. Prociv P. Strongyloidiasis in the Northern Territory. Medical Journal of Australia 1993; 159: 636-637.
9. Prociv P, Luke R. Observations on strongyloidiasis in Queensland Aboriginal communities. Medical Journal of Australia 1993; 158: 160-163.
10. Lim L, Biggs BA. Fatal disseminated strongyloidiasis in a previously treated patient. Medical Journal of Australia 2001; 174: 355-356.
11. Grove DI. Clinical manifestations. In: Strongyloidiasis, a major roundworm infection of man. London: Taylor & Francis, 1989; 155-173.
12. Marti H, Haji HJ, Savioli L et al. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted Helminth infections in children. American Journal of Tropical Medical Hygiene 1996; 55: 477-481.
13. Toma H, Sato Y, Shiroma Y, Kobayashi J, Shimabukuro I, Takara M. Comparative studies on the efficacy of three anthelminthics on treatment of human strongyloidiasis in Okinawa, Japan. Southeast Asian Journal of Tropical Medical Public Health 2000; 31: 147-151.
14. Gann PH, Neva FA, Gam AA. A randomized trial of single- and two-dose ivermectin versus thiabendazole for treatment of strongyloidiasis. Journal of Infectious Diseases 1994; 169: 1076-1079.
15. Lahn MM, Staub-Schmidt T, Him R et al. Strongyloides stercoralis infection in a non-immunosuppressed tourist with involvement of the central nervous system. Tropical and Geographical Medicine 1994; 46: 368-370. www.harrisonsonline.com/server-java/Arknoid/harrisons/1096-7133

