Breast cancer is the most common type of cancer among women and the leading cause of cancer deaths worldwide. In 2008, approximately 1.38 million new cases and 458 400 deaths from breast cancer accounted for 14% of all cancer deaths1. In most low- and middle-income countries (LMCs), the incidence breast cancer is rising faster than in developed countries, where rates are already high2. In Brazil, 52 680 new cases of breast cancer were predicted for 20123.
Breast cancer early detection methods include breast self-examination (BSE), clinical breast examination (CBE), ultrasound (US), mammography and MRI. Recent publications have found that BSE does not contribute to reducing mortality, and women should know the risks and benefits4-6 of this screening method. Clinical breast examination is recommended by the American Cancer Society; nevertheless the US Preventive Services Task Force reported insufficient evidence for or against recommending CBE as a screening method4. The Canadian National Breast Cancer Screening Trial argued that the inclusion of CBE in an organized screening program contributes very little to early detection7. Mammography has been used in most developed countries as the gold standard for breast cancer screening, accounting for an approximate 30% reduction in mortality8,9.
It should be noted that these screening techniques (BSE, CBE, US, mammography and MRI) have largely been studied in developed countries, in the context of robust healthcare infrastructures where there are frequent encounters between patients and care providers, and many women have their cancers detected at an early stage. However, in countries such as Brazil most mammography screening is opportunistic, and the decentralized healthcare system and lack of systematic patient documentation makes it difficult to evaluate the quality of screening programs10.
Study setting
The city of Barretos is situated in southeastern Brazil and the northern state of São Paulo, with an area of 1564 km2. Barretos' total population is 112 101 with 3.7% living in the rural area of the municipality, outside the urban geographical perimeter11.
In the Barretos region, mammograms can be performed in the private sector, public sector, or at Barretos Cancer Hospital (BCH; a non-profit cancer foundation); however, all diagnosed cases are treated at BCH, the only oncology hospital in the region. In 2003, BCH commenced implementation of the first organized screening program for breast cancer in Brazil, utilizing both mobile and fixed units12,13.
The objective of this study was to evaluate the difficulties of an organized screening program for breast cancer in a developing country, with emphasis on the methods used in relation to the stage of disease at diagnosis.
This prospective study included all 248 women (aged 40-69 years) who were diagnosed with breast cancer between January 2007 and December 2009, and who lived in one of the 19 cities of the Regional Health Department V (RHD-V), one of the 17 RHDs in São Paulo State. Regional Health Department V is an administrative area in northwestern São Paulo State, which encompasses 19 cities14, including both the rural and urban areas of Barretos city. This RHD has an estimated population of 54 238 women between the ages of 40-69 years.
In 2003, BCH was been contracted by RHD-V to implement a breast cancer screening program in the region. The program was established with two mammography units in the hospital and a mobile unit offering 120 examinations per day free of charge. Details of this program have been described previously12. During the study period, the BCH Screening Program diagnosed 2747 new cases of breast cancer in women from numerous Brazilian regions, among them 248 from the public health system and private sectors of RHD-V.
All women diagnosed by BCH who accepted the invitation to participate in the study signed informed consent, and were interviewed in their homes (by prior telephone appointment), or while at BCH during a follow-up visit. The interview was conducted with the aid of a pre-prepared questionnaire which contained sociodemographic questions, and general healthcare and preventive breast cancer screening questions. The questionnaire included some questions about breast cancer screening in the Barretos region. Independent variables were the methods used to make the diagnosis of breast cancer and the stage of the disease. Dependent variables were the women's sociodemographic characteristics, access to the health system and symptoms.
To ensure patient privacy, the only people present at the interview were the interviewer, the patient, and one companion (if desired). In this study women were considered asymptomatic if they had no complaint of a lump in the breast or nipple discharge. Patients with a second primary breast cancer, those who did not agree to participate in the survey, and those whose questionnaires were incomplete were excluded from the study. All survey results were anonymous.
Pathology and follow-up data, such as staging and treatment information, were compiled from patients' medical records held by BCH's Medical Records and Statistics Service. The staging of lesions was performed according to UICC TNM Classification of Malignant Tumors15.
All statistical analyzes were performed using SPSS for Windows v17.0 (www.spss.com). Descriptive statistics were reported and the significance of differences between categories were evaluated using a two-sided χ2 test. When necessary, Fisher's exact test was performed. Statistical significance was considered for p-values lower than 0.05 (α=5%). Univariate and multivariate analyses were also performed for predictors of CS at breast cancer screening.
Ethics approval
This project was approved by the BCH's Institutional Review Board (#364/2010).
Of 248 women living in one of the 19 cities in RHD-V who were diagnosed with breast cancer between 2007 and 2009, 206 (83.1%) participated in this study. Forty-two patients were excluded for the following reasons: 22 were deceased, six had previous breast cancers, five had moved to the area only for treatment, four had moved to another state, three were not at the address provided, and two women declined to participate in the study.
Most women in this sample (96.1%) lived in an urban area, 68 (33.0%) were aged 40 to 49 years and 121 (58.7%) had no breast cancer symptoms at the time of diagnosis. Their educational level was predominantly low with 67.5% having 0-8 years of schooling. All 206 women were referred for treatment at BCH (Table 1).
Table 1: Sociodemographic and preventive breast cancer screening
characteristics of 206 women diagnosed with breast cancer in the Barretos region
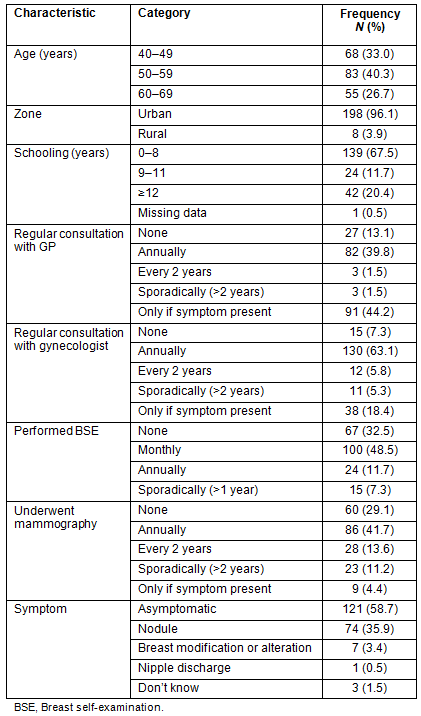
Among the 206 women interviewed in this study, the mammography screening program conducted by BCH was responsible for 95 (46.1%) diagnoses, 49 (23.8%) of which were made at the hospital unit and 46 (22.3%) in the mobile unit. Of the remaining 111 women, 49 (23.8%) were referred to BCH from the public health system and 62 (30.1%) from the private system, and their referral was due to suspected breast cancer noted on mammography performed outside the BCH Screening Program.
One hundred twenty-seven (61.7%) women used the public health system for doctor visits, examination, and treatment. The health behaviors of these women, such as periodic consultations with doctors and gynecologists, are described (Table 1).
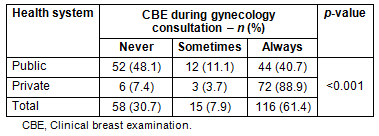
Clinical breast examination was performed regularly in 61.4% of gynecological consultations, with a statistically significant difference (p<0.001) in CBE frequency between doctors in the public and private health systems (Table 2). Nonetheless, there was no difference in the percentage of cancers diagnosed by CBE in the public versus private health sectors (p=0.097).
Table 2: Frequency of clinical breast examination
performed by public health system doctors
compared with those working in the private health system
Breast self-examination was reported to be performed monthly by 48% of women. Mammography was performed at least every 2 years in 55.3% of women, as recommended (Table 1). Slightly more than half the women (116; 56.3%) were able to answer that a woman should start mammography screening at the age of 40.
Non-adherence to mammography recommendations (women who had never undergone mammography or had mammograms performed at intervals of >2 years) was found in 92 (44.7%) women. The most frequent reason for this was 'negligence and/or laziness' (39.5%), with other reasons including a lack of understanding that mammography was aimed at early detection in asymptomatic women rather as than a tool for women with symptoms or physical examination findings (38.2%), and avoidance of screening due to fear of the disease (11.8%). Practical barriers, such as difficulty in obtaining access to mammography, were reported by only 5.6% of women.
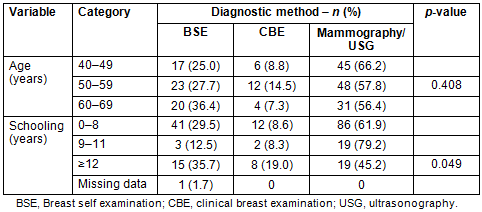
Mammography was responsible for making the diagnosis of breast cancer in 112 women (54.4%), followed by 60 (29.1%) diagnosed by BSE, 22 (10.7%) by CBE and 12 (5.8%) by ultrasound. This distribution was similar across all age groups (p=0.408) and statistically different in women with according to years of schooling (p=0.049; Table 3). Of the 12 cancers diagnosed by US, 10 (83.3%) were performed in private clinics.
Table 3: Distribution of diagnostic methods for
breast cancer according to age group and years of school
Overall 58.7% of the women in this study were asymptomatic and three (1.5%) could not answer questions regarding their symptoms. Among those from the BCH Screening Program, 69.5% were asymptomatic, compared with 55% from the private and 45.8% public systems. Among asymptomatic women participating in the BCH Screening Program, 70.8% had CS 0-I disease in comparison with 58.1% in the private and 50% in the public systems. Detection of early breast cancer (CS 0-I) was more frequent among the asymptomatic women (p<0.001) and is associated with the diagnostic method (p<0.001). The distribution of staged lesions according to age, diagnostic methods, and symptoms is shown (Table 4).
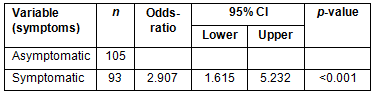
The logistic regression analysis with all variables showed that asymptomatic women were more likely to be diagnosed with early breast cancer (OR=2.907; 95% CI=1.615-5.232; p<0.001; Table 5).
Table 4: Distribution of lesions in stages according
sociodemographic characteristics, diagnostic methods and symptoms
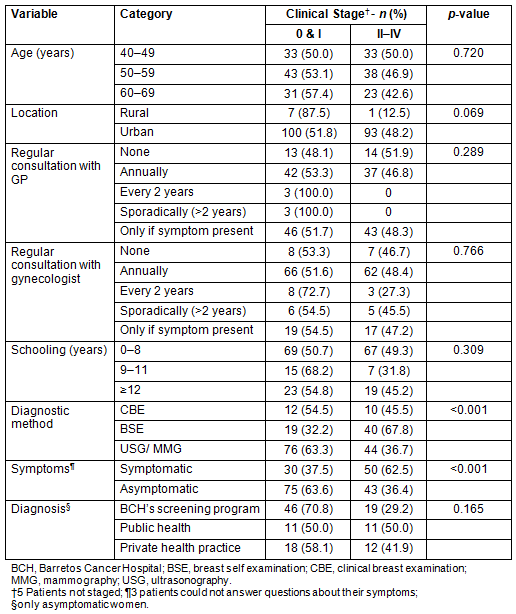
Table 5: Results of multivariate analysis for breast cancer clinical stage
Discussion
In Brazil and other developing countries, mammography screening programs are mostly opportunistic rather than used to screen asymptomatic women16,17. The Brazilian National Cancer Institute (INCA) formerly recommended that all women between 40 and 49 years have CBE, and women aged 50 to 69 years have a mammogram every 2 years18. However, a 2009 Federal Law mandated that women aged 40 years and older have annual mammograms19. This created controversy among physicians and health administrators concerned about how to implement a breast cancer screening program that initiates screening at this minimum age.
The 2003 initiation of BCH's breast cancer prevention program aimed for the implementation of an organized mammography screening program, with the understanding that this would be integrated into healthcare infrastructure that included the education of women regarding the importance of early detection of breast cancer, and offered affordable and readily available mammography. In addition to this, and equally important, was the intention that treatment programs would also be available and accessible. It was hoped that this successful pilot program would be disseminated to other areas of Brazil and other developing countries20-22.
One of the greatest problems in implementing a formal cancer screening program in Brazil is that there is no public database with the names and addresses of the target population. In addition, in this and several other regions of Brazil, a transient population migrates from the poorest states for several months annually for agricultural work, especially manual sugar cane cutting23. This floating population poses problems for disease prevention and overloads the local health system. Because these workers have no fixed addresses it is difficult to locate them to arrange participation in a screening program. These difficulties, combined with the limited public and private access to the breast cancer screening, require greater efforts to define the target population.
It is critical that the region's screening programs offer quality, free specialized care for breast cancer because the population is of predominantly low educational level and socioeconomic status, therefore suffering difficulty in gaining access to public health.
In this study it was observed that 39.8% of 206 women diagnosed with breast cancer had symptoms when the diagnosis was made. Of the 95 women who underwent mammography through BCH's mammography screening program mobile or hospital-based units, 29 (30.5%) were symptomatic, indicating that the mammography is not serving the purpose it was designed for: to detect small clinically occult cancers. Instead it was merely a healthcare system access facilitator.
In Brazil, even assuming CBE could contribute to the early diagnosis and a favorable outcome for breast cancer patients, such breast examinations were not always performed, particularly in the public sector. This may be a reflection of physicians' low wages in the public health system. One-third of São Paulo state physicians work for more than 60 hours a week and another third work for four or more different employers24. This is suggested by the women's responses to questions about CBE during gynecological consultations, and also by the number of potential cases identified by this method. In 38.6% of consultations with gynecologists, CBE was not performed, and this was more frequent among women consulting doctors in the public health system (p<0.001). Only 22 women (10.7%) reported that CBE was responsible for detecting their cancer.
Studies from the USA have shown higher rates of referral for breast cancer screening among obstetricians and gynecologists, compared with other physician types25. Thus, the involvement of gynecologists in breast cancer prevention programs must be considered. Apart from that, incentive programs may increase other physicians' performance in this area, as there is evidence of the effectiveness of this strategy in a 1990 report from England where payments for cervical cytology incentivized the vast majority of doctors to reach higher performance levels26.
An alternative is to involve non-medical professionals from the Family Health Program, a Brazilian program which benefits approximately 96.1 million people (50.7% of the population)27. This Brazilian Ministry of Health program consists of multidisciplinary teams that are responsible for monitoring a certain number of families located within a set geographical area. The teams develop strategies for health promotion, preventive care, recovery and rehabilitation for diseases and other disorders, as well as for maintaining the health of their community28. The Community Health Agent (CHA) members of these programs must reside in their neighborhoods of operation and provide understandable information to people in need. In 2010, a total of 234 767 CHAs in Brazil attended 60.9% of the population27. A recent study demonstrated that these professionals are of key importance to the success of a screening program in Brazil29.
Among the present study participants, 48.5% performed BSE regularly; however, this practice was more common among women with less schooling (68.3%) and those dependent on the public health system (63.3%). A BSE screening was responsible for 29.1% of the women who discovered their own cancer, yet 45.5% of them had CS II-IV at diagnosis. A recent study suggests that low-cost tests such as the BSE and CBE could be used to improve survival in LMCs, and should be more immediately achievable through increased awareness of breast cancer and the potential for successful treatment30. In the multivariate analysis, the presence of symptom was the dependent variable and this was strongly associated with advanced breast cancer (OR=2.907; 95% CI=1.615-5.232; p<0.001). However, the large number of patients with CS II-IV shows the importance of educating and training women in BSE, particularly in regions where mammography is not readily available.
The practice of BSE is controversial and two studies have demonstrated no reduction in breast cancer mortality using this screening method6,31, although it has been suggested that in developing countries it may play a role in improving outcomes32-36. In Brazil the National Cancer Institute (INCA) recommends BSE to be included in health education to help improve a woman's knowledge of her own body, and that BSE be used in association with CBE performed by a physician for early detection of breast cancer18.
This study found that 92 (44.7%) women had either never had a mammogram or underwent mammography less frequently than recommended. This was mostly due to ignoring healthcare recommendations, lacking knowledge about mammography, or having a fear of breast cancer. These findings are consistent with several studies on barriers to the performance of mammography in LMCs, and in some subgroups of the population of developed countries; although, in the study setting, availability or inability to pay were not found to be factors37-41. Although 25% of the population in the study region were living below the poverty line42 only 5.3% of women reported economic or access issues. This is likely to be due to the multiple community intervention strategies and ease of access to the free hospital and mobile units of BCH's Prevention Program13.
The participating patients' distribution of clinical stages of disease at diagnosis was compared with cancer data from the State of São Paulo Cancer Hospital Records (FOSP), which receives data from 76 institutions in the state of São Paulo, including public and private, as well as cancer and university hospitals. There was a statistically significant reduction in advanced breast cancer cases in asymptomatic women in the Barretos Screening Program (p<0.001), where 8.1% of breast cancer cases were diagnosed as CS III and IV compared with 33.4% in the rest of the state during the same period43. Among the asymptomatic women diagnosed during this screening program, 70.8% had CS 0-I, which is significantly higher (p<0.001) than the 30.9% seen in the rest of the state43. These findings are consistent with those reported in developed countries where mammography screening has been performed for more than two decades9.
Limitations and strengths
This study's results may be not representative of the actual breast cancer situation nationwide due to the sample being a population from small region of Brazil. Despite the high proportion of the women participating (83.1%), not all could be interviewed. For most (22 or 8.9%) this was due to death from the disease, and only two women declined to participate.
Achieving improved breast cancer outcomes in such settings has been a challenge worldwide. Even in small, developed countries participation in cancer screening programs often do not exceed 50% of the target population44. The authors believe their success was because the program reported included an intensive community intervention aspect which may have increased adherence to recommendations for breast care, and the fact that the program was constructed according to the three pillars:
- Bringing affordable health care and screening to locations close to the women.
- Having an identification and recall program.
- Affordable and effective treatment being available.
The reported program also used experienced professionals to interpret mammograms and had an international partnership with an institution with significant experience in organized breast cancer screening, which helped to improve the quality of processes45. Finally, it was critical to have the support and commitment of the administrators of the authors' institutions.
While this project is a work in progress, efforts at early breast cancer detection and treatment have been improved. Many challenges remain but the data gathered in this study provide considerable information to guide future efforts. These data suggest that such an approach is feasible and can potentially improve breast cancer outcomes for many women. It is hoped that this will also assist the development of similar programs in other areas of Brazil and other developing countries.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. A Cancer Journal for Clinicians 2011; 61(2): 69-90.
2. Anderson BO. Understanding social obstacles to early breast cancer detection is critical to improving breast cancer outcome in low- and middle-resource countries. Cancer 2010; 116(19): 4436-4439.
3. Brasil. Ministério da Saúde. Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA). Coordenação Geral de Ações Estratégicas. Coordenação de Prevenção e Vigilância. Estimativa 2012: incidência de câncer no Brasil. Rio de Janeiro: INCA, 2011.
4. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2009; 151(10): 727-737, W237-742.
5. Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. A Cancer Journal for Clinicians 2010; 60(2): 99-119.
6. Thomas DB, Gao DL, Ray RM, Wang WW, Allison CJ, Chen FL et al. Randomized trial of breast self-examination in Shanghai: final results. Journal of the National Cancer Institute 2002; 94(19): 1445-1457.
7. Miller AB, To T, Baines CJ, Wall C. Canadian National Breast Screening Study-2: 13-year results of a randomized trial in women aged 50-59 years. Journal of the National Cancer Institute 2000; 92(18): 1490-1499.
8. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database of Systemic Reviews 2006; 4: CD001877.
9. US Preventive Services Task Force (USPSTF). Screening for breast cancer: US Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2009; 151(10): 716-726, W-236.
10. Bihrmann K, Jensen A, Olsen AH, Njor S, Schwartz W, Vejborg I et al. Performance of systematic and non-systematic ('opportunistic') screening mammography: a comparative study from Denmark. Journal of Medical Screening 2008; 15(1): 23-26.
11. IBGE. Instituto Brasileiro de Geografia e Estatística: cidades. (Online) 2012. Available: http: //www.ibge.gov.br/cidadesat/painel/painel.php?codmun=350550 (Accessed 5 January 2012).
12. Mauad EC, Nicolau SM, Moreira LF, Haikel RL Jr, Longatto-Filho A, Baracat EC. Adherence to cervical and breast cancer programs is crucial to improving screening performance. Rural Remote Health 9(3): 1241. (Online) 2009. Available: www.rrh.org.au (Accessed 2 April 2013).
13. Mauad EC, Silva TB, Haikel RL Jr, Bauab S, Longatto-Filho A. Is community intervention in breast cancer screening in Brazil feasible? Journal of Medical Screening 2011; 18(1): 51.
14. Brasil, Governo do Estado de São Paulo. Secretaria da Saúde. DRS V - Barretos. (Online) 2013. Available: http://www.saude.sp.gov.br/ses/institucional/departamentos-regionais-de-saude/drs-v-barretos (Accessed 16 April 2013).
15. Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of malignant tumors, 7th edn. Hoboken, NJ: John Wiley, 2009.
16. Marchi AA, Gurgel MS. [Adherence to the opportunistic mammography screening in public and private health systems]. Revista Brasileira de Obstetrícia e Ginecologia 2010; 32(4): 191-197.
17. Puschel K, Coronado G, Soto G, Gonzalez K, Martinez J, Holte S et al. Strategies for increasing mammography screening in primary care in Chile: results of a randomized clinical trial. Cancer Epidemiology, Biomarkers Prevention 2010; 19(9): 2254-2261.
18. Instituto Nacional do Câncer (INCA). Controle do Câncer de Mama - Documento do Consenso. (Online) 2004. Available: http: //www.inca.gov.br/publicacoes/Consensointegra.pdf (Accessed 23 February 2009).
19. Presidência da República. Lei nº 11.664, de 29 de abril de 2008. In: Subchefia para Assuntos Jurídicos (Eds). Brasília: Casa Civil; 2008.
20. Otto SJ, Fracheboud J, Looman CW, Broeders MJ, Boer R, Hendriks JH et al. National Evaluation Team for Breast Cancer Screening. Initiation of population-based mammography screening in Dutch municipalities and effect on breast-cancer mortality: a systematic review. Lancet 2003; 361(9367): 1411-1417.
21. Shapiro S, Coleman EA, Broeders M, Codd M, de Koning H, Fracheboud J et al. Breast cancer screening programmes in 22 countries: current policies, administration and guidelines. International Breast Cancer Screening Network (IBSN) and the European Network of Pilot Projects for Breast Cancer Screening. International Journal of Epidemiology 1998; 27(5): 735-742.
22. Sasieni P. Evaluation of the UK breast screening programmes. Annals of Oncology 2003; 14(8): 1206-1208.
23. Moraes MAFD, Figueiredo MG, Oliveira FCR. Migração de trabalhadores na lavoura canavieira paulista: uma investigação dos impactos sócio-econômicos nas cidades de Pedra Branca, Estado do Ceará, e de Leme, Estado de São Paulo. Revista de Economia Agrícola 2009; 56(2): 21-35.
24. Carvalhaes C. O médico brasileiro: A realidade do médico no Brasil. São Paulo: Conselho Regional de Medicina do Estado de São Paulo (CREMESP). (Online) 2005. Available: http: //www.cremesp.org.br/?siteAcao=Noticias&id=1077 (Accessed 20 April 2011).
25. Bhosle M, Samuel S, Vosuri V, Paskett E, Balkrishnan R. Physician and patient characteristics associated with outpatient breast cancer screening recommendations in the United States: analysis of the National Ambulatory Medical Care Survey Data 1996-2004. Breast Cancer Research and Treatment 2007; 103(1): 53-59.
26. Silcock J, Ratcliffe J. The 1990 GP contract--meeting needs? Health Policy 1996; 36(2): 199-207.
27. Brasil. Ministério da Saúde. Cadastro Nacional de Estabelecimentos de Saúde (CNES). Brasília, DF: Secretaria de Atenção à Saúde (DATASUS); 2011. Available: http: //dab.saude.gov.br/abnumeros.php .
28. Brasil. Ministério da Saúde. Programa Saúde da Família. Brasília/DF: Ministério da Saúde, 2011. Available: http: //portal.saude.gov.br/portal/saude/cidadao/area.cfm?id_area=149 .
29. Mauad EC, Nicolau SM, Gomes UA, da Costa Vieira RA, de Castro Mattos JS, Longatto-Filho A et al. Can mobile units improve the strategies for cervical cancer prevention? Diagnostic Cytopathology 2010; 38(10): 727-730.
30. Shulman LN, Willett W, Sievers A, Knaul FM. Breast cancer in developing countries: opportunities for improved survival. Journal of Oncology 2010; 1-6.
31. Semiglazov VF, Moiseenko VM, Manikhas AG, Protsenko SA, Kharikova RS, Popova RT et al. [Interim results of a prospective randomized study of self-examination for early detection of breast cancer (Russia/St.Petersburg/WHO)]. Vopr Onkol 1999; 45(3): 265-271.
32. Loh S, Packer TL, Yip CH, Passmore A. Targeting health disparity in breast cancer: insights into women's knowledge of their cancer profile in Malaysia. Asian Pacific Journal of Cancer Prevention 2009; 10(4): 631-636.
33. Lopez-Carrillo L, Suarez-Lopez L, Torres-Sanchez L. [Breast cancer examination in Mexico: summary of the results from the National Survey of Reproductive Health]. Salud Publica Mexico 2009; 51(Suppl 2): s345-349.
34. Rasu RS, Rianon NJ, Shahidullah SM, Faisel AJ, Selwyn BJ. Effect of educational level on knowledge and use of breast cancer screening practices in Bangladeshi women. Health Care Women International 2011; 32(3): 177-189.
35. Tara S, Agrawal CS, Agrawal A. Validating breast self examination as screening modalities for breast cancer in eastern region of Nepal: a population based study. Kathmandu University Medical Journal 2008; 6(1): 89-93.
36. Yip CH, Smith RA, Anderson BO, Miller AB, Thomas DB, Ang ES et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer 2008; 113(8 Suppl): 2244-2256.
37. Martin-Lopez R, Hernandez-Barrera V, De Andres AL, Garrido PC, De Miguel AG, Garcia RJ. Breast and cervical cancer screening in Spain and predictors of adherence. European Journal of Cancer Prevention 2010 19(3): 239-245.
38. Ko CM, Sadler GR, Ryujin L, Dong A. Filipina American women's breast cancer knowledge, attitudes, and screening behaviors. BMC Public Health 2003; 3: 27.
39. Sadler GR, Ko CM, Cohn JA, White M, Weldon RN, Wu P. Breast cancer knowledge, attitudes, and screening behaviors among African American women: the Black cosmetologists promoting health program. BMC Public Health 2007; 7: 57.
40. Buki LP, Jamison J, Anderson CJ, Cuadra AM. Differences in predictors of cervical and breast cancer screening by screening need in uninsured Latino women. Cancer 2007; 110(7): 1578-1585.
41. Puschel K, Thompson B, Coronado G, Gonzalez K, Rain C, Rivera S. 'If I feel something wrong, then I will get a mammogram': understanding barriers and facilitators for mammography screening among Chilean women. Family Practice 2010; 27(1): 85-92.
42. Rocha SMRd. Pobreza no Brasil: afinal, de que se trata? 3rd edn. Rio de Janeiro: FGV, 2007.
43. Fundação Oncocentro de São Paulo (FOSP). Acesso ao Banco de Dados - RHC. São Paulo: FOSP, 2011. Available: http: //200.144.1.68/cgi-bin/dh?rhc/rhc-geral.def.
44. Autier P, Shannoun F, Scharpantgen A, Lux C, Back C, Severi G et al. A breast cancer screening programme operating in a liberal health care system: the Luxembourg Mammography Programme, 1992-1997. International Journal of Cancer 2002; 97(6): 828-832.
45. Deras IL, Aubin SM, Blase A, Day JR, Koo S, Partin AW et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. Journal of Urology 2008; 179(4): 1587-1592.
