More than 35 million people worldwide are living with dementia1. This number is projected to increase to 100 million by 2050, with a corresponding estimated global dementia-related cost of $US604 billion (2010 projection)1. Clearly, the prevalence of dementia will have significant health, social, and economic impacts in the 21st century. Of particular concern are the considerable socioeconomic consequences of dementia caused by the extent and duration of dementia-related disability, cost of care, and loss of productivity of those living with dementia and their caregivers1. Dementia is now viewed as a chronic disease, similar to diabetes, HIV, and multiple sclerosis. It is a protracted condition that can be controlled, but cannot be cured. As a result, countries across the globe are calling for a population health and a chronic disease management strategy approach to dementia, comprising early intervention and treatment, risk reduction, and self-management2-5.
Self-management focuses on maximizing function through biological, psychological, and social interventions, and encompasses engaging in various activities and daily life organization in order to manage one's condition and symptoms, protect and promote one's health and wellness, and maintain as active a life as possible6,7. In response to the call for a chronic disease management strategy approach to dementia, 'living well' with dementia and 'supported self-care' frameworks are being adopted by dementia advocacy and volunteer organizations. With early interventions becoming increasingly recognized as essential for individuals with dementia, more and more attention is being directed towards health and wellness promotion as a critical component for 'living well' with dementia, particularly for individuals in the earlier stages of the disease8. For example, the Alzheimer's Society of Canada and the Alzheimer's Foundation of America recommend a healthy diet, mental exercise, physical activity, socialization, participating in spiritual and religious activities, stress management, and other lifestyle choices, such as controlling hypertension, as valuable self-management strategies for maintaining overall health for those living with dementia9,10.
It is thought that health and wellness promotion information provided soon after dementia diagnosis, along with enhanced coping skills, may lead to health behavior changes that will prevent excess disability or premature loss of function and institutionalization. Not only do health and wellness promotion interventions have the potential to affect overall function, they may also affect community life11. To date, very little has been published regarding self-management for people with dementia. Participating in support groups that emphasize social activities and sharing experiences with peers, as self-management strategies, have been shown to be beneficial for people with dementia12. A 2011 report on self-management found that people with dementia do engage in a variety of physical activities, such as hockey, golf, biking, hiking, skiing, running, fitness courses, swimming, and walking, and cognitive exercises including playing card games, computer games, and attending memory programs at the local Alzheimer's society13. Despite the shift to self-management and encouraging health and wellness promotion behaviors in people with dementia, there is a paucity of research and very little is known about the types of behaviors those with dementia engage in to self-manage their health and wellness. The purpose of this exploratory study was to (1) describe the health- and wellness-related self-management behaviors patients attending a rural and remote memory clinic engage in and (2) examine the relationship between engaging in health and wellness behaviors and psychological function, neuropsychological function, independence in daily activities, and physical function (gait and balance).
An exploratory mixed-methods study of a cross-sectional sample was used to examine health and wellness behaviors, and the relationship between health and wellness behaviors, specifically engaging in exercise and nutrition (eating well), and measures of function, depression, memory, and gait and balance.
Sample
The sample included 260 patients referred to the Rural and Remote Memory Clinic (RRMC), Saskatoon, Saskatchewan, Canada. The RRMC is an interdisciplinary (neurology, neuropsychology, nursing, physical therapy), one-day, 'one-stop' clinic, which has been in existence since 2004. The RRMC integrates telehealth for assessment and follow-up and serves community-dwelling rural and remote residents, specifically those living more than 100 km from tertiary care14,15. Referrals to the RRMC are made by family physicians to the neurologist, but may be initiated by a family member or any member of the health team14. The clinic follows a family-centered approach. At least one caregiver attends the RRMC with the patient to provide information on the patient's history and daily functioning, to participate in joint interviews, and to complete questionnaires if and as required14. The RRMC uses a multidisciplinary approach and established guidelines for the clinical diagnosis of dementia. The added value of this multidisciplinary assessment is the ability to differentiate among the dementia subtypes16. The interprofessional assessment includes up-to-date blood work, a CT head scan, neuropsychological assessment, physiotherapy assessment, neurology assessment, clinical history, and an extensive caregiver interview14.
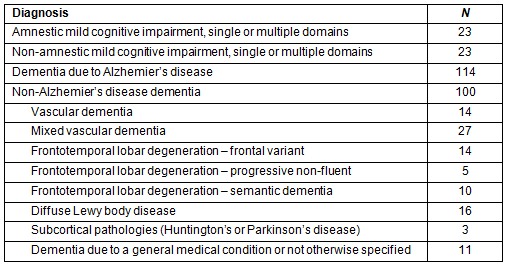
All patients in the sample were diagnosed with a cognitive impairment resulting from single-domain or multi-domain amnestic (aMCI), non-amnestic mild cognitive impairment (non-aMCI), dementia due to Alzheimer's disease (AD), or non-AD dementia (various etiologies). Not surprising, almost half of the sample (43.8%) had a diagnosis of AD. Summaries and frequencies of diagnoses of the patient sample are shown in Table 1.
Table 1: Patient sample: frequency of diagnosis based on best-practice interprofessional assessment
Measures
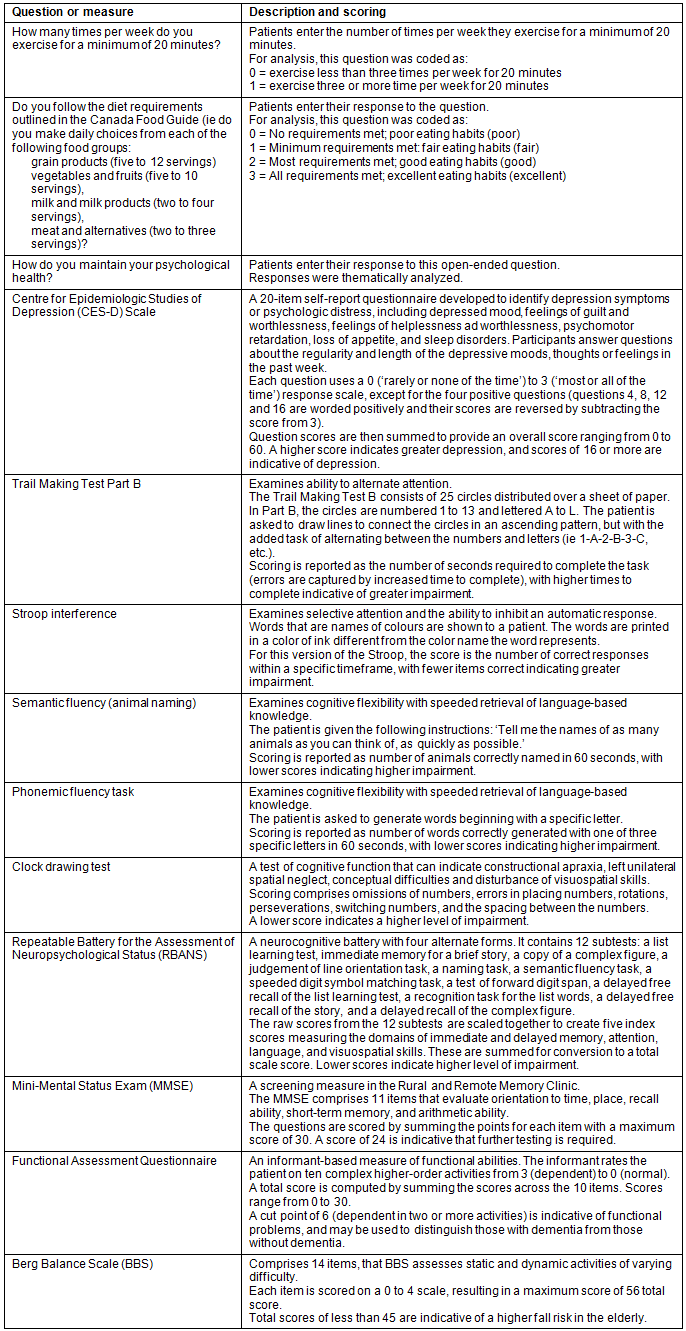
Patient and caregiver questionnaires and assessments administered at the RRMC clinic day appointment14 provided the sociodemographic data including age, sex, years of formal education, and health and wellness behaviors and lifestyle data. Regarding exercise and nutrition (eating well) behaviors, patients answered two specific questions: 'How many times per week do you exercise for a minimum of 20 minutes?' and 'Do you follow the diet requirements outlined in the Canada Food Guide [Canada's Food Guide to Healthy Eating]?'17. Canada's Food Guide to Healthy Eating provides general information about the number of servings from each food group - grain products (5-12 servings), vegetables and fruit (5-10), milk products (2-4) , and meat and alternatives (2-3) that Canadians should consume17. Canada's Food Guide to Healthy Eating has a total diet approach to choosing foods. The total diet approach aimed to meet both energy and nutrient requirements, recognizing that energy needs vary based on age, body size, activity level, sex and specific conditions, such as pregnancy and breastfeeding17. Regarding psychological health and wellbeing behaviors, patients answered an open-ended question: 'How do you maintain your psychological health?'
Patients completed questions from the Centre for Epidemiologic Studies of Depression Scale18. Patients also completed a neuropsychological battery. The ability to alternate attention was measured with the Trail Making Test Part B19. The ability to inhibit an automatic response was measured with the Stroop interference test20. Cognitive flexibility with speeded retrieval of language-based knowledge was measured using animal naming, a semantic fluency task21, and a phonemic fluency task22. The clock drawing test measured visual construction, abstraction, and inhibition23. Finally, the index scores from the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)24 were analyzed, which included a measure of attention and speed of mental processing, visuospatial and visuoconstructional abilities, language abilities of naming and generative fluency, and immediate and delayed memory for word lists and a short story, and delayed recall of a complex figure. The Mini-Mental Status Exam(MMSE)25 is used as a screening measure in the RRMC, and is completed at the beginning of the neuropsychological battery. Caregivers who accompanied patients to the clinic reported on the patients' ability to function independently in daily life (instrumental activities of daily living) with the Functional Assessment Questionnaire26. The Berg Balance Scale (BBS)27, a 14-item task-oriented test that assesses balance abilities, was quantified from the physical therapy assessment. Table 2 further describes each of the questions and measures above, scoring methods, and cut-offs as applicable.
Table 2: Questions and measures used for data collection at the Rural and Remote Memory Clinic
Data analysis
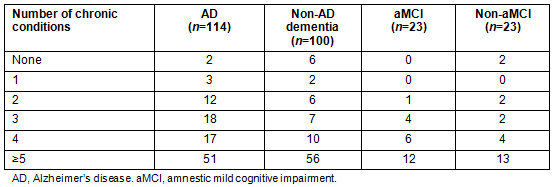
Patients were grouped based on their diagnoses. As well as an AD dementia group, a heterogeneous group of non-AD dementia (as per the diagnostic breakdown in Table 1) and two groups of patients with MCI were created - one for aMCI, which is a risk factor for dementia due to AD, and one for non-aMCI. Questionnaire data were analyzed using descriptive statistics and correlational analyses. Bivariate associations were assessed using point-biserial and Spearman's correlations. It was postulated that patients' number of chronic conditions would pose a confound with these associations and so the number of chronic conditions across the four diagnostic groups - aMCI, non-aMCI, dementia due to AD and non-AD dementia (Table 3) - was examined. As can be seen in the first row of Table 4, these small associations were statistically non-significant; therefore, the number of chronic conditions was not considered a covariate in any analyses. Due to small cell sizes in the two MCI groups, inferential analyses were only conducted for the two dementia groups (AD and non-AD dementia).
Coding for the questions 'How many times per a week do you exercise for a minimum of 20 minutes?' and 'Do you follow the diet requirements outlined in the Canada Food Guide?' are reported in Table 2. Canada's Food Guide to Healthy Eating was updated in 2007, 3 years after the implementation of the RRMC, thus the authors analyzed the cases where responses were provided to the Canada's Food Guide to Healthy Eating17 question. Associations between self-reported exercise with psychological and neuropsychological, functional, and gait and balance variables were assessed for the AD versus non-AD dementia diagnostic groups. Associations between self-reported adherence to Canada's Food Guide to Healthy Eating17 with psychological, neuropsychological, functional, and gait and balance were also assessed for the AD and non-AD groups. To examine the self-reported strategies used to maintain psychological health, responses to this open-ended question were analyzed thematically. Thematic analysis encompasses searching across a data set to find and analyze repeated patterns (themes), and does not derive from nor is tied to a particular epistemological or theoretical position28,29. Specifically, the authors used a semantic approach whereby the themes are identified within the surface meaning of the data, without looking beyond what is written29.
Written responses were read and re-read by two authors individually to capture theme frequencies and co-occurrences. Similarities, patterns, and consistency of the themes were reviewed; this process was repeated until consensus was reached regarding the themes, and the theme reflected the written responses as a whole. Last, the qualitative themes were quantized to transform them into quantitative data30. Qualitative data were first reduced into items (themes) intended to mean only one thing, so that the items could then be represented numerically30. Specifically, the developed themes were tallied using descriptive analyses to determine the frequency counts, and then these tallies were summarized into general categories.
Table 3: Frequency of chronic conditions by diagnostic group
Ethics approval
The research was approved by the University of Saskatchewan Behavioural Research Ethics Committee (BEH #03-12-19).
Participants were aged between 44 and 97 years (mean=74.07, standard deviation (SD)=9.18) and had between 0 and 20 years of formal education (mean=10.48, SD=3.30). Almost two-thirds (61.5%) of the sample was female. Mini-Mental Status Exam scores ranged from 5 to 30 (mean=23.25, SD=4.50).
For those with dementia due to AD the mean MMSE was 21.58 (SD=4.07), and for those with non-AD dementia the mean MMSE was 23.08 (SD=4.65). For those with aMCI, the mean MMSE was 27.22 (SD=1.86) and for those with non-aMCI the mean MMSE was 27.95 (SD=1.56). As expected, there was a statistically significant difference between groups, with the dementia groups having lower MMSE scores than MCI groups (F (3237)=24.14, p<0.001). Mean RBANS total scores ranged from 45 to 103 (mean=66.51, SD=13.07). For the AD dementia group the mean RBANS score was 61.16 (SD=10.09) and for the non-AD dementia group the mean RBANS score was 66.03 (SD=13.73). For the aMCI group the mean RBANS score was 77.41 (SD=7.67) and for the non-aMCI group the mean RBANS was 78.79 (SD=12.26). Again, as expected, there was a significant difference between groups (F(3182)=20.23, p<0.001, with the dementia groups having lower RBANS scores than MCI groups.
Table 3 shows the frequency counts of chronic diseases for the aMCI, non-aMCI, dementia due to AD and non-AD dementia diagnostic groups. Almost half (45.8%) of those with dementia due to AD reported having five or more chronic conditions. For those with non-AD dementia, 56% reported having five or more chronic conditions. A similar pattern was seen with the MCI diagnostic groups, with 52.2% of those with aMCI reporting five or more chronic conditions and 56.2% of those with non-aMCI reporting five or more chronic conditions.
For the non-AD dementia diagnostic group, associations between exercise and tests of neuropsychological function were trivial or small and statistically non-significant, likely due to the heterogeneity of the group. In contrast, for the group with AD, engagement in exercise for 20 minutes for three or more times a week was moderately associated with improved performance on the Stroop interference test and better balance, as indicated by BBS scores. For both the AD and non-AD dementia groups, engagement in regular exercise was not significantly associated with caregivers' reports of patients' ability to function in daily life (small effect), and there was a trivial and statistically non-significant association between exercise and symptoms of depressed mood.
Associations between nutritional practices and the neuropsychological, mood, and functional variables were statistically non-significant for the non-AD group. For the group of patients with an AD diagnosis, better nutritional practices were associated with improved scores on the RBANS language subtests (naming and semantic fluency subtests) and the total RBANS score, and better clock drawing and phonemic fluency test scores. Although the sample size was small, the moderate association between the BBS was significant and better nutrition practices was associated with better balance, as measured by the BBS (Table 4).
Table 5 illustrates exercise and nutrition habits for the aMCI, non-aMCI, dementia due to AD and non-AD dementia diagnostic groups. Except for the AD group, more than half of the other groups - aMCI (57.1%), non-aMCI (60%) and non-AD dementia (52.9%) - reported exercising for 20 minutes less than three times per week. Less than half (42.7%) of the AD group reported exercising for 20 minutes less than three times per week. Importantly, over a third (39.4%) of the total sample reported not exercising at all on a weekly basis and the vast majority (97.5%) reported weekly exercise less than 150 minutes weekly, the minimum amount for adults and older adults according to the Canadian Society for Exercise Physiology31. Of the 229 respondents with complete questionnaire data, about three-quarters (74%) of individuals reported they met most or all of the Canada's Food Guide to Healthy Eating dietary recommendations17. Three-quarters (75.2%) of the AD group, 73.8% of the non-AD dementia group, 73.9% of the aMCI group and 66.7% of the non-aMCI (60%) group reported meeting most or all of the Canada's Food Guide to Healthy Eating dietary recommendations17.
More than two-thirds (71%) of respondents indicated they were engaged in wide variety of activities to maintain their psychological health (Table 6). Many of the 187 participants who reported engaging in activities to maintain their psychological health reported more than one activity. However, a very small number (2.1%) reported engaging in a combination of physical activities, social activities, and mentally stimulating activities. About 20% of the sample reported engaging in passive activities or did not engage in any activities to maintain their psychological health. The types of activities the sample reported engaging in to maintain psychological health are depicted in Figure 1.
Table 4: Associations between lifestyle variables of regular exercise (higher score coded for more exercise) and nutrition habits (higher score coded for reported greater adherence to Canada's Food Guide to Healthy Eating) for neuropsychological, functional, physical, and mood measures for the dementia due to AD or non-AD dementia groups
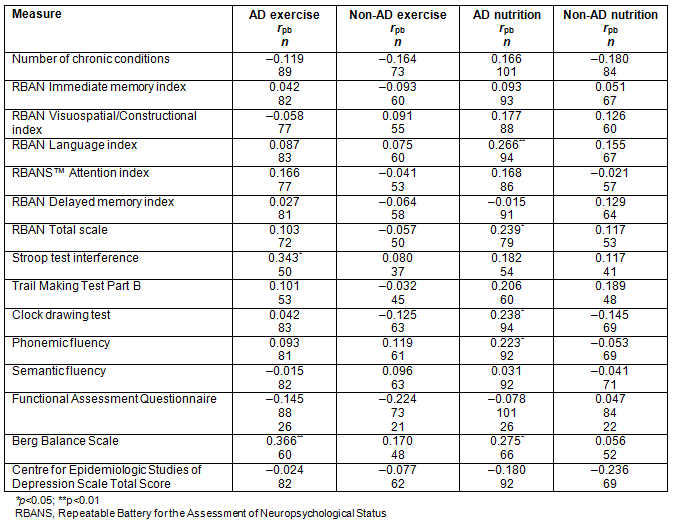
Table 5: Frequency of exercise and nutrition health-related behaviors of the patient sample
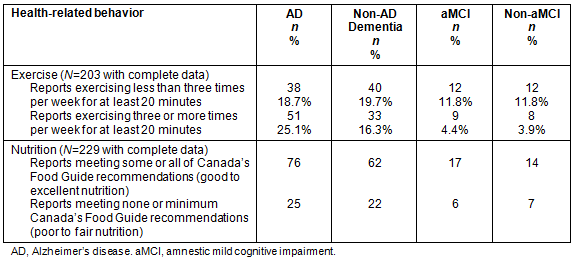
Table 6: Frequency of patients' responses to the open-ended question 'How do you maintain your psychological health?' (N=187)
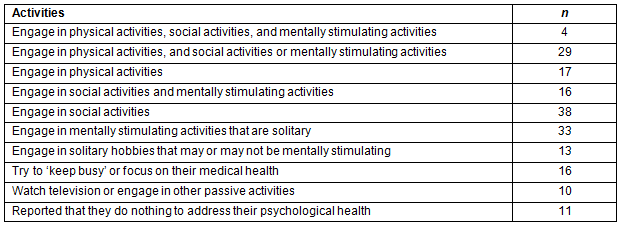
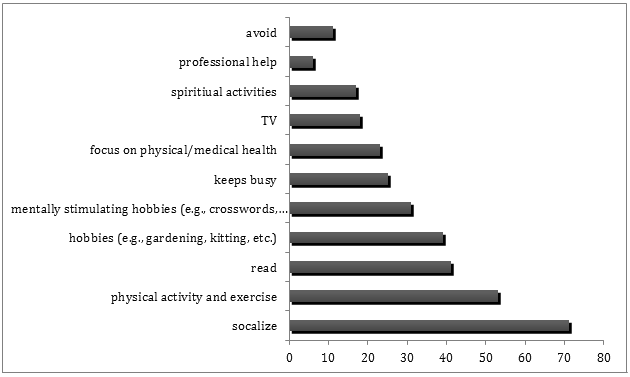
Figure 1: Tally of all the activities reported in response to the open ended question 'How do you maintain your psychological health?' (Some of the 187 respondents reported up to four different activities, and all activities were included in these tallies.)
Discussion
This exploratory study examined the health and wellness behaviors of patients attending a rural and remote memory clinic and the relationship between engaging in health and wellness behaviors and psychological function, neuropsychological function, independence in daily activities, and balance. Almost half of those with dementia due to AD, and over half of those with non-AD dementia and aMCI reported having five or more chronic conditions. Considering risk of dementia increases with increasing age, with prevalence increasing from 8% in those 65 years and older to 34.5% in those 85 years and older32, and that the majority of older persons experience multiple comorbid conditions and diseases33, this result is not surprising. However, there are implications for chronic disease approaches and self-management strategies for those with dementia. Those with dementia may not only have to deal with their dementia diagnosis, but may have self-management needs related to multiple co-existing comorbid chronic conditions that need to be considered in any health and wellness promotion intervention or program.
The Alzheimer's Society of Canada and the Alzheimer's Foundation of America recommend the following self-management strategies for maintaining overall health for those living with dementia: a healthy diet; mental exercise; physical activity; socialization; participation in spiritual and religious activities; stress management; and other lifestyle choices, such as controlling hypertension9,10. The majority of the sample of rural and remote dwelling people with dementia reported participating in a variety of pleasurable activities to maintain psychological health and report good nutrition habits. However, only a very small percentage reported participating in a combination of physical activities, social activities and mentally stimulating activities, about a tenth of the sample was not actively engaged in addressing their psychological health at all or reported engaging only in passive activities, and about a quarter reported meeting minimal or none of Canada's Food Guide to Healthy Eating17 recommendations. The importance of cognitive stimulation in the prevention and treatment of early-stage memory loss34, and the importance of socializing for addressing psychological health12, has been reported in the literature. Because of the cross-sectional sample and the nature of the data collection and analyses, it was not possible to discern why these individuals were not engaging in positive, health promoting behaviors, and this requires further investigation. In addition, the findings suggest that more targeted strategies need to be developed in the RRMC to ensure patients and caregivers are made aware of the importance of engaging in a range of health and wellness promotion activities.
Interestingly, reporting good nutritional habits was associated with better neuropsychological function, specifically total RBANS scores, RBANS23 language sub-test scores, clock drawing test scores and phonemic fluency scores, and BBS scores for the AD subsample. There is a dearth of research related to nutrition in the earlier stages of dementia; however, authors have reported that education regarding nutrition may have a positive effect on patients with respect to cognitive function35, and meeting nutritional needs is an important aspect of caring for people with dementia, in all stages including the early stage of disease36. Epidemiological studies suggest that nutrition can modify the risk of dementia onset37,38. A diet high in vegetables, legumes, fruits, cereals, and unsaturated fatty acids has been found to be associated with decreased risk of developing mild cognitive impairment and of the conversion of mild cognitive impairment to AD39, and there is evidence that nutritional interventions can decrease brain atrophy in people with mild cognitive impairment40. Unfortunately, the subsample of aMCI and non-aMCI was too small for further inferential analyses to examine associations between reported nutritional habits and neuropsychological function and balance. Despite the growing evidence about the benefits of healthy eating, the research on nutrition in people with dementia has tended to focus on those in the later stages of the disease. Little attention, until more recently, has been directed towards the importance of good nutrition for those in the earlier stages of dementia. This warrants further investigation, particularly the benefits of healthy eating in the early stages as part of 'living well' with dementia.
Although positive benefits of exercise were found in those with AD, half of the sample who reported on exercise (all subgroups) reported exercising less than three times per week for 20 minutes, and many reported not exercising for the recommended time for health benefits31. In addition, over half of the aMCI subgroup reported exercising less than three times per week for 20 minutes. A feasibility study of high-intensity aerobic exercise in 33 individuals with aMCI found that women in the aerobic exercise group demonstrated improved performance on multiple tests of executive function, and for men aerobic exercise had a favorable effect only on Trail Making Test Part B performance41. The authors suggest that aerobic exercise plays a protective role by attenuating progression of cognitive symptoms in MCI41. Similarly, a 12-month randomized controlled trial of moderate-intensity walking for adults with MCI found that attention and memory improved for women; for men, only the most adherent had improved memory, but attention was unchanged42.
Rural residents often have fewer destinations within walking distance, and a lack of well-maintained sidewalks can create a fear of injury that discourages walking or biking43. The built environment in rural and remote areas can also positively or negatively influence individuals' attitudes towards health and health behaviors44. A qualitative examination of physical activity in older adults living in rural areas concluded that built environment characteristics (eg well-maintained sidewalks, accessible destinations, and an aesthetically pleasing environment) were each associated with increased walking and biking45. Whether or not the built environment was an important factor related to physical activity and exercise participation for this RRMC patient sample warrants further investigation.
Currently, there are no published guidelines specifically targeting people with dementia, and particularly those in the earlier stages of dementia. The data suggest there is a small but significant association between exercising three or more times per week for at least 20 minutes and better balance when measured objectively, and better Stroop interference scores in the subsample with AD. Both of these findings are consistent with literature in older adults without dementia. Older adults who exercise are more likely to have better balance46, and a beneficial impact of physical activity and exercise has been found on older adult executive functioning47. According to the Canadian Society for Exercise Physiology's Canadian Physical Activity Guidelines, older adults (65 years and older) should engage in at least 150 minutes of moderate-to-vigorous-intensity physical activity per week31. It is also recommended that Canadians should try to exceed the minimum activity thresholds, as there are greater health benefits with more variety, intensity and duration of the physical activity31.
Similarly to good nutrition, physical exercise appears to reduce risk of dementia48. Although few high-quality randomized control trials exist regarding exercise interventions for people with dementia49, best-practice guidelines based on cumulative evidence suggest that individualized exercise programs may improve fitness, cognition, and day-to-day function for people with mild to moderate dementia50. In addition, there is evidence that physical activity at an intensity level adapted to a person's overall physical capacities should be promoted as part of a 'healthy lifestyle' for older individuals with memory loss51. The exercise-related findings, as well as the psychological health findings, speak to the need for additional education for the RRMC patients related to 'living well' with dementia and the need for more formalized self-management programs specifically targeting those living in rural and remote areas. Health is a considered 'a complete state of physical, mental and social well-being and not merely the absence of disease or infirmity'52. Early diagnosis and interventions are critical elements of effectively managing people with dementia, and promoting health and wellness behaviors in people with dementia has become recognized as a key component of the overall management of this patient population. Health and wellness promotion, prevention and early intervention activities have the potential to not only reduce the prevalence and impact of a number of chronic diseases that individuals with dementia may have, but may also prevent or slow the onset and progression of dementia. As dementia is now recognized as a chronic disease, similar to those with other chronic diseases, engaging in 'living well' activities may help those with dementia remain active and may positively influence function and quality of life.
There is a dearth of information and research related to health and wellness promotion for people with dementia, and services for people in early stages of dementia are often limited; as a result these individuals frequently become isolated and stigmatized53. A 2007 expert panel consensus report found that support groups for older adults in the early stages of dementia comprised poorly designed research designs and had weak scientific evidence34. Well-designed self-management interventions based on strong evidence may address the care gap and may be a possible solution for providing support in the early stages of disease54. The goal of self-management is to improve quality of life, and self-management focuses on providing patients with the skills to manage their lives in the face of chronic illness6,7. Self-management programs typically include information about common problems faced by individuals with a chronic condition, and address topics such as nutrition, exercise, managing medications, psychological health, and management of symptoms. However, the nature of dementia is considered a barrier for self-management and highlights the need for early diagnosis. A majority of people with dementia considered that information provided following diagnosis was aimed predominantly at their carers, leading to feelings of powerlessness and helplessness55,56. The findings from this emerging work can help inform memory clinics, such as the RRMC, about the best approaches to providing important health and wellness information to patients and caregivers, and can assist with the design of targeted education and self-management programs.
This study found that rural and remote dwelling people with dementia and mild cognitive impairment attending a memory clinic do participate in a variety of pleasurable activities to maintain psychological health and report good nutrition habits. However, a majority do not engage in recommended amounts of weekly exercise, and only a few participate in a combination of physical activities, social activities and mentally stimulating activities. The effects of the rural environment, as potential barriers to engaging in health and wellness behaviors, should be further explored. Engaging in exercise and good nutritional habits were found to have beneficial effects in the sub-sample of patients with AD. Patients and their caregivers attending the RRMC may require additional education and information and more targeted strategies regarding beneficial health and wellness promoting behaviors related to their diagnosis and concurrent comorbid conditions.
Acknowledgements
This research was supported by an Applied Chair in Health Services and Policy Research from the Canadian Institutes of Health Research and the Saskatchewan Health Research Foundation (D. Morgan).
References
1. Prince M, Bryce R, Ferri C. Alzheimer's Disease International: world Alzheimer report 2011 - the benefits of early diagnosis and intervention (Online). 2011. Available: http://www.alz.co.uk/research/WorldAlzheimerReport2011/pdf (Accessed 12 June 2013).
2. Alzheimer's Australia. Towards a national dementia preventative health strategy. (Online). Available: http://www.fightdementia.org.au/common/files/NAT/20100800_Nat_NP_21TowNatDemPrevHlthStrat.pdf (Accessed 12 June 2013).
3. Sian MS. Chronic disease management programme on dementia. Singapore Family Physician 2011; 37(3)(Supp 1): 30-41.
4. Alzheimer's and Related Disorders Society of India. The dementia India report: prevalence, impact, costs and services for dementia. Executive summary. (Online) 2010. Available: http://www.alzheimer.org.in/dementia_ex_2010.pdf (Accessed 12 June 2013).
5. European Conference on the Fight Against Alzheimer's Disease and Related Afflictions. The fight against Alzheimer's disease and related disorders. (Online) 2008. Available: http://www.plan-alzheimer.gouv.fr/IMG/pdf/Alzheimer_conference_EN_081030.pdf (Accessed 13 May 2013).
6. Bodenheimer T, Lorig K, Holman H, Grumbach, K. Patient self-management of chronic disease in primary care. Journal of the American Medical Association 2002; 288(19): 2469-2475.
7. Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet 2004; 364: 1523-1537.
8. Burgener SC, Buettner LL, Beattie E, Rose KM. Effectiveness of community-based, nonpharmacological interventions for early-stage dementia: conclusions and recommendations. Journal of Gerontological Nursing 2009; 35(3): 50-57.
9. Alzheimer's Foundation of America. Brain health: lifestyle choices. (Online) 2012. Available: http://www.alzfdn.org/BrainHealth/successfulaging.html (Accessed 30 March 2013).
10. Alzheimer's Society of Canada. Heads up for healthier living. For people with Alzheimer's disease and their families. (Online) 2011. Available: http://www.alzheimer.ca/~/media/Files/national/Heads-up/Heads_Up_Healthier_Living_2012_e.ashx (Accessed 30 March 2013).
11. Zarit SH, Femia EE, Watson J, RiceOeschger L, Kakos B. Memory club: a group intervention for people with early-stage dementia and their care partners. The Gerontologist 2004; 44: 262-269.
12. Mather L. Memory Lane Cafe: follow-up support for people with early stage dementia and their families and carers. Dementia: The International Journal of Social Research and Practice 2006; 5(2): 290-293.
13. Centre for Education and Research on Aging & Health. Self-management of dementia: final summary report. (Online) 2011. Available: http://akeontario.editme.com/files/HealthyLiving/Academic%20Summary.FINAL_.pdf (Accessed 16 June 2013).
14. Morgan D, Crossley M, Kirk A, D'Arcy C, Stewart N, Biem J, et al. Improving access to dementia care: development and evaluation of a rural and remote memory clinic. Aging & Mental Health 2009; 13(1): 17-30.
15. Morgan D, Crossley M, Kirk A, McBain L, Stewart N, D'Arcy C, et al. Evaluation of telehealth for pre-clinic assessment and follow-up in an interprofessional Rural and Remote Memory Clinic. Journal of Applied Gerontology 2011; 30(3): 304-333.
16. Wolfs CA, Dirksen CD, Severens JL, Verhey FR. The added value of a multidisciplinary approach in diagnosing dementia: a review. International Journal of Geriatric Psychiatry 2006; 21(3): 223-232.
17. Health Canada. Canada's Food Guide to Healthy Eating. Ottawa, ONT: Health Promotion and Programs Branch, Minister of Public Works and Government Services Canada, 1992.
18. Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiological Studies-Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and Aging 1997; 12: 277-287.
19. Reitan RM. Trail Making Test: manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory, 1992.
20. Trenerry M, Crosson B, DeBoe J, Leber W. The Stroop neuropsychological screening test. Odessa, FL: Psychological Assessment Resources, 1989.
21. Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 2nd edn. Philadelphia: Lea & Febiger, 1983.
22. Spreen O, Benton AL. Neurosensory Center comprehensive examination for aphasia. Victoria, BC: University of Victoria, Neuropsychology Laboratory, 1977.
23. Tuokko H, O'Connell M. A review of quantified approaches to the qualitative assessment of clock drawing. In: A Poreh (ed). The quantified process approach to neuropsychological assessment. New York: Taylor & Francis, 2006.
24. Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology 1998; 20(3): 310-319.
25. Folstein M, Folstein S, McHugh P. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975; 12: 189-198.
26. Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology 1982; 37: 323-329.
27. Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Canadian Journal of Public Health 1992; 83(Suppl 2): S7-S11.
28. Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology 2006; 3(2): 77-101.
29. Boyatzis RE. Transforming qualitative information:thematic analysis and code development. Thousand Oaks, London & New Delhi: SAGE Publications, 1998.
30. Tashakkori A, Teddlie C. Mixed methodolgy: combining qualitative and quantitative approaches. Thousand Oaks, CA: Sage Publications, 1998.
31. Canadian Society for Exercise Physiology. Canadian physical activity guidelines. (Online) 2011. Available: http://www.csep.ca/CMFiles/Guidelines/CSEP_Guidelines_Handbook.pdf (Accessed 6 June 2013).
32. McDowell I, Hill G, Lindsay J, Helliwell B, Costa L, Beattie BL, et al. Canadian Study of Health and Aging: study methods and prevalence of dementia. Canadian Medical Association. 1994; 150(6): 899-912.
33. World Health Organization. The global burden of disease: 2004 update. Geneva: World Health Organization, 2008.
34. Burgener S, Beattie E, Bossen A, Buckwalter K, Buettner L, Fick D, et al. Consensus report: review of scientific evidence addressing prevalence, documented needs, and interdisciplinary research: persons in early stage Alzheimer's dementia. Chicago, IL: Alzheimer's Association, 2007.
35. Rivière S, Gillette-Guyonnet S, Voisin T, Reynish E, Andrieu S, Lauque S, et al. A nutritional education program could prevent weight loss and slow cognitive decline in Alzheimer's disease. Journal of Nutrition, Health and Aging. 2001; 5: 295-299.
36. Holm B, Söderhamn O. Factors associated with nutritional status in a group of people in an early stage of dementia. Clinical Nutrition 2003; 22(4): 385-389.
37. Xu W L, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology 2011; 76(18): 1568-1574.
38. Morris MC. Nutritional determinants of cognitive aging and dementia. Proceedings of the Nutrition Society 2012; 71(1): 1-13.
39. von Arnim CA, Gola U, Biesalski HK. More than the sum of its parts? Nutrition in Alzheimer's disease. Nutrition 2010; 26(7-8): 694-700.
40. Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PloS ONE 2010; 5(9): 1-10.
41. Baker L, Frank LL, Foster-Schubert K, Green PS, Wilkinson CS, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of Neurology 2010; 67(1): 71-79.
42. van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. British Journal of Sports Medicine 2008; 42(5): 344-351.
43. Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychological Medicine 2008; 39: 3-11.
44. Forbes D, Forbes S, Morgan DG, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database of Systematic Reviews 2008, Issue 3, art. no. CD006489. DOI: 10.1002/14651858.CD006489.pub.2.
45. Hogan DB, Bailey P, Carswell A, Clarke B, Cohen C, Forbes D, et al. Management of mild to moderate Alzheimer disease and dementia. Alzheimer's Disease & Dementia 2007; 3: 355-384.
46. Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database of Systematic Reviews 2011, Issue 11, art. no. CD004963. DOI: 10.1002/14651858.CD004963.pub3.
47. Hogan DB, Bailey P, Black S, Carswell A, Chertkow H, Clarke B, et al. Diagnosis and treatment of dementia: 5. Nonpharmacologic and pharmacologic therapy for mild to moderate dementia. Canadian Medical Association Journal 2008; 179: 1019-1026.
48. Department of Health. Living well with dementia: a national dementia strategy. London: Department of Health, 2009.
49. Strath S, Isaacs R, Greenwald MJ. Operationalizing environmental indicators for physical activity in older adults. Journal of Aging & Physical Activity 2007; 15(4): 412-424.
50. U.S. Department of Health and Human Services. Obesity and the built environment. (Online) 2004. Available: http://grants.nih.gov/grants/guide/rfa-files/RFA-ES-04-003.html (Accessed 23 March 2013).
51. Bollman RD, Clemenson HA. Structure and change in Canada's rural demography: an update to 2006. (Online) 2007. Available: http://www.statcan.gc.ca/pub/21-006-x/21-006-x2007007-eng.htm (Accessed 23 March 2013).
52. World Health Organization. WHO definition of health. Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference, New York, 19-22 June, 1946; signed on 22 July 1946 by the representatives of 61 States (Official Records of the World Health Organization, no. 2, p. 100) and entered into force on 7 April 1948. (Online) 2003. Available: http://who.int/about/definition/en/print.html(Accessed 6 April 2013).
53. Wlodarczyk JH, Brodaty H, Hawthorne G. The relationship between quality of life, mini-mental state examination, and the instrumental activities of daily living in patients with Alzheimer's disease. Archives of Gerontology and Geriatrics 2004; 39(1): 25-33.
54. Fitzsimmons S, Buettner LL. Health promotion for the mind, body, and spirit: a college course for older adults with dementia. American Journal of Alzheimer's Disease and Other Dementias 2003; 18(5): 282-290.
55. Mountain GA. Self-management for people with early dementia: an exploration of concepts and supporting evidence. Dementia 2006; 59(3): 429-446.
56. Mountain GA, Craig CL. What should be in a self-management programme for people with early dementia? Aging and Mental Health 2012; 16(5): 576-583.


