Introduction
Chronic conditions including cardiovascular disease and type 2 diabetes mellitus contribute an estimated 80% to the significant gap between Indigenous and non-Indigenous life expectancy in Australia. These conditions appear to have been rare or non-existent prior to European colonisation1. Despite the efforts of the Close the Gap campaign, chronic conditions continue to increase in Indigenous communities in Australia2,3. Intergenerational social inequity and economic disadvantage underpin the rise in chronic conditions because they impact all aspects of life, including health-related behaviours, and access to health and other services1,4. Furthermore, an imposed food system has resulted in a rapid change for Australian Indigenous peoples from the traditional lifestyle – characterised by high levels of physical activity and a moderate energy, nutrient-dense diet – to a more ‘westernised’ lifestyle, characterised by a high-calorie, nutrient-poor, processed diet and low physical activity5,6.
Traditional risk factors for these chronic conditions are those identified from the Framingham Heart Study and include smoking, total cholesterol, low high-density lipoprotein (HDL) cholesterol, systolic blood pressure (BP) and diabetes7. These risk factors have been reported to be more prevalent in Indigenous Australians compared to non-Indigenous Australians8; however, risk of future coronary heart disease in Indigenous Australians has been significantly underestimated when only traditional risk factors were included in risk prediction models9. Hence, additional risk factors need to be considered in determining risk. These include body composition and body fat distribution, albuminuria, dyslipidaemia (high triglycerides and low HDL cholesterol), impaired fasting glucose and impaired glucose tolerance. Socioeconomic and environmental structural and psychosocial factors also play a significant role in increasing risk of chronic disease in Indigenous populations8.
The first nationwide survey of chronic condition risk factors prevalence among Indigenous Australians was recently undertaken in 2012/13, but no follow-up data describing change in risk factors are available yet10. Prior to this national survey, the only information on risk factors for chronic conditions were from a small number of surveys among selected, mostly remote, Indigenous communities11-21, with very few studies reporting temporal changes in risk factors22-24. Thus, there is a critical gap in knowledge regarding the follow-up of chronic condition risk factors in Indigenous Australians, particularly in relation to community-wide health promotion campaigns.
The Healthy Lifestyle project25 was established in one remote community in Arnhem Land, in the Northern Territory in Australia, to improve community-led health promotion initiatives that focused on healthy eating and physical activity to prevent diabetes and related chronic conditions25. Arnhem Land is a region of about 79 000 km2 in the north-eastern part of the Northern Territory, with a largely remote Indigenous population (approximately 12 000).
Findings from the baseline survey showed low body mass index (BMI) and absence of diabetes among those aged less than 30 years, but higher BMI and high prevalence of diabetes among middle-aged participants. These findings informed a program of health promotion initiatives that were developed in partnership with community, health and government agencies, and aimed to maintain leanness among younger people and delay the onset of diabetes and related conditions. To measure the impact of these initiatives, a second follow-up survey was undertaken. The authors now report on changes in risk factors between the baseline and follow-up surveys. The aims were to (i) compare risk factor prevalence from two cross sectional surveys at baseline and at follow-up, and (ii) perform a longitudinal analysis of change in risk factors among participants who attended both surveys.
Methods
Study population/participants
In response to community-wide concerns about the increasing impact of chronic diseases, the Healthy Lifestyle project was established as a joint program between a local community organisation and Menzies School of Health Research25. It was the first community-wide screening for chronic condition risk factors in this remote centralised community in north-eastern Arnhem Land, and focused on type 2 diabetes and cardiovascular disease prevention. Methods for the baseline survey have been previously described12. In brief, all Indigenous residents aged 15 years or more were invited to participate using several recruitment methods. The first survey was conducted between September 2001 and March 2002 and follow-up data collected between July 2003 and July 2004. Median time between baseline and follow up was 1.9 years (698 days).
Between the first and second surveys, 131 discrete community-based cardiovascular disease and diabetes prevention activities were implemented over 3 years by more than 20 different organisations, including the local health centre, Menzies School of Health Research, government and community agencies as previously reported25. Many of the activities were repeated across multiple years, which meant that more than 200 activities were undertaken25. In order to promote a community-directed approach, the prevention activities were based on existing community activity, which was identified by a network of community members and researcher facilitators, and then supported and strengthened. Examples of activities included employing and training community project officers, physical activity workshops, workshops and materials for community gardens, a yearly week-long healthy lifestyle festival and improvements in fruit and vegetable display and quality at the local store.
A community census of residents aged 15 years or more was conducted in April 2002. Of the 706 residents, 378 (54%) participated in the baseline survey. In terms of sex distribution, participants were representative of the census population; however, older women were over-represented12. For the present analysis, survey data were available for 294 participants at baseline, and for 218 participants at the second survey. Of these, 87 attended both surveys and had longitudinal data on risk factors. Participants were excluded if they were pregnant (n=7 at baseline, n=3 at follow-up), and thus 425 participants contributed data to this analysis.
Measures
Local Aboriginal researchers administered a health questionnaire in local language and undertook the clinical examinations with other members of the research team. The same protocol was used for the baseline and follow-up surveys. Height, weight, waist circumference, hip circumference and BP were measured. Self-reported smoking was recorded12.
Blood samples were obtained for measurement of glucose and lipids after an overnight fast and analysed at a central laboratory (Flinders Medical Centre, Adelaide, South Australia) accredited in the US Centers for Disease Control Lipid Standardisation Program. Haemoglobin A1c (HbA1c) was assayed using cation exchange high-performance liquid chromatography using a Pharmacia MonoS column; and insulin by immunoassay (Abbott Axsym). Spot early-morning urine samples were collected to determine urinary albumin-to-creatinine ratio (ACR)12.
Statistical analysis
Diabetes was classified as HbA1c ≥48 mmol/mol (6.5%)26,27 or if a medical record confirmed diabetes. Impaired glycaemia was defined as HbA1c of 39–48 mmol/mol (5.7–6.5%)26. Hypertension was defined as systolic BP≥140mmHg and/or diastolic BP≥90mmHg28. High cholesterol was defined as ≥5.5 mmol/L, high triglycerides as ≥2.0 mmol/L and low HDL cholesterol ≤1.0 mmol/L. Microalbuminuria was defined as ACR≥3 mg/mmol and <30 mg/mmol; macroalbuminuria was defined as ≥30 mg/mmol29. BMI was classified into four groups: <20 kg/m2, 20–<25 kg/m2, 25–<30 kg/m2 and ≥30 kg/m230. Elevated waist-to-hip ratio (WHR) was defined as ≥0.90 for men and ≥0.85 for women30.
To analyse change in risk factors, multilevel mixed-effects logistic regression was used for categorical variables and multilevel mixed-effects linear regression for continuous variables. These methods took into account that the follow-up included some repeat participants from baseline as well as new participants. HbA1c, fasting glucose, triglycerides, ACR and WHR were not normally distributed and thus were log-transformed before analysis. Models were adjusted for age and sex. Change in prevalence of risk factors was stratified according to three age groups (15–29 years, 30–49 years and >50 years) and sex. Change in HbA1c was stratified into those with diabetes and those without diabetes. Longitudinal change of risk factors among those individuals who attended both surveys was also assessed using similar regression techniques. Statistical analysis was performed using Stata v14.0 (StataCorp; http://www.stata.com).
Ethics approval
Participants provided informed consent and the study was approved by the Joint Human Research Ethics Committee of the Northern Territory Department of Health and Community Services and Menzies School of Health Research (HREC 00-63) following approval from the Aboriginal sub-committee of the HREC, which has veto rights over applications involving Indigenous participants. The local Community Council, who comprise Indigenous Elders, endorsed the study in 2001, and the authors worked in collaboration with researchers.
Results
Cross-sectional analysis
In the first survey, 53% of participants were women and the mean (range) age was 35 (15–75) years. In the second survey, 56% of participants were women and mean (range) age was 40 (15–76) years. Between surveys, self-reported smoking prevalence rates were stable but high (57% in survey 1 and 56% in survey 2, unadjusted, Table 1). When smoking prevalence was stratified by age and sex, smoking tended to increase for women although confidence intervals overlapped, but it remained stable in men for all age groups (Fig1).
The median HbA1c increased from 35 mmol/mol (5.4%) at the first survey to 37 mmol/mol (5.5%) in the second survey (unadjusted, Table 1), which showed borderline significance after adjusting for age and sex (p=0.047) (Table 2). The magnitude of increase in the geometric mean of HbA1c was the same in the younger and older age groups, although this change was only significant in the younger age group (p=0.037) after adjusting for sex. Significant age-adjusted increases in the geometric mean of HbA1c were observed in women (p=0.019), not men (p=0.786). When the cohort was stratified into those with and without diabetes, there was no increase in the age- and sex-adjusted geometric mean of HbA1c in those without diabetes (from 39 to 41 mmol/mol (5.4% to 5.4%), p=0.339), and a significant increase in those with diabetes (from 57 to 63 mmol/mol (7.5% to 8.1%), p=0.021). The prevalence of diabetes was similar between the first survey (19%) and the second survey (20%); however, impaired glucose tolerance increased from 18% to 22% (Table 1).
Table 2 presents the change in risk factors after adjusting for age and sex. Although there was a significant increase in total cholesterol, there was no significant change in the prevalence of high total cholesterol. There was a small but significant increase in HDL cholesterol levels, and a significant decrease in the prevalence of low HDL cholesterol levels. Triglyceride levels significantly increased, but prevalence of high triglycerides did not change. These results varied between sexes and age groups (Fig1). As expected, the prevalence of high cholesterol increased with age for both men and women. The prevalence of low HDL cholesterol decreased in younger men (from 47% to 34%), women aged 31–50 years (from 74% to 66%), older men (from 72 to 56%) and older women (from 80% to 52%) but it remained stable in younger women (from 47% to 48%) and men in the middle age group (31–50 years) (from 57% to 53%) (Fig1).
Mean BMI and WHR remained essentially unchanged between baseline and follow-up (Table 2). The prevalence of a high WHR increased in the younger age group in men (from 19% to 41%) and women (from 65% to 73%) but decreased in the older age group in men (from 100% to 81%) and women (from 95% to 74%) (Fig1).
Mean ACR levels and prevalence of albuminuria were similar for the first and second surveys (Tables 1,2). Although systolic and diastolic BP were similar in the first and second surveys, the prevalence of hypertension significantly reduced (odds ratio (OR)=0.31, p=0.019).
Table 1: Cohort characteristics at first and second surveys: the Healthy Lifestyle Study 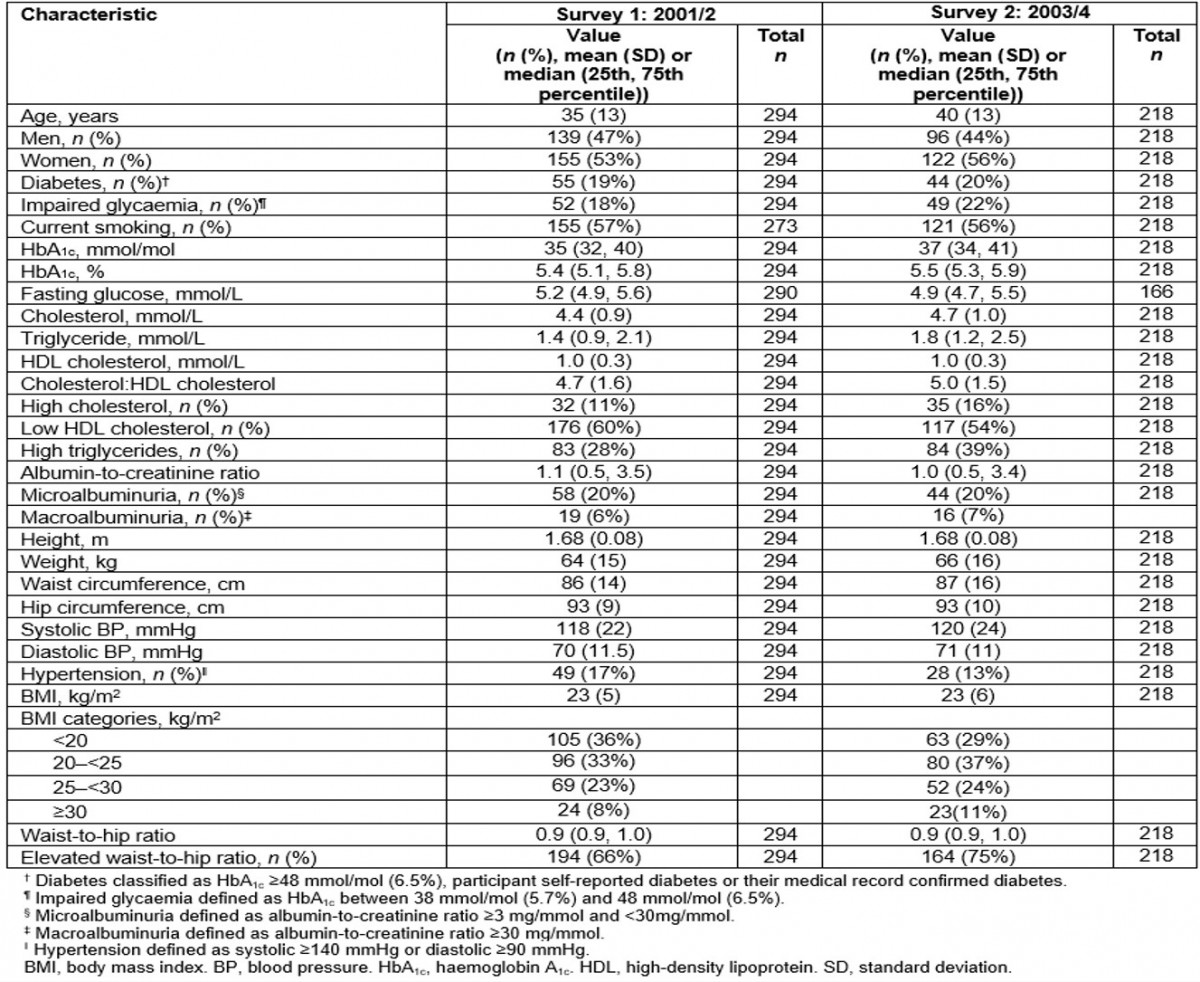
Table 2: Change in chronic disease risk factors between first and second surveys: the Healthy Lifestyle Study (Data are coefficient for continuous variables and odds ratios for categorical variables. All models adjusted for age and sex)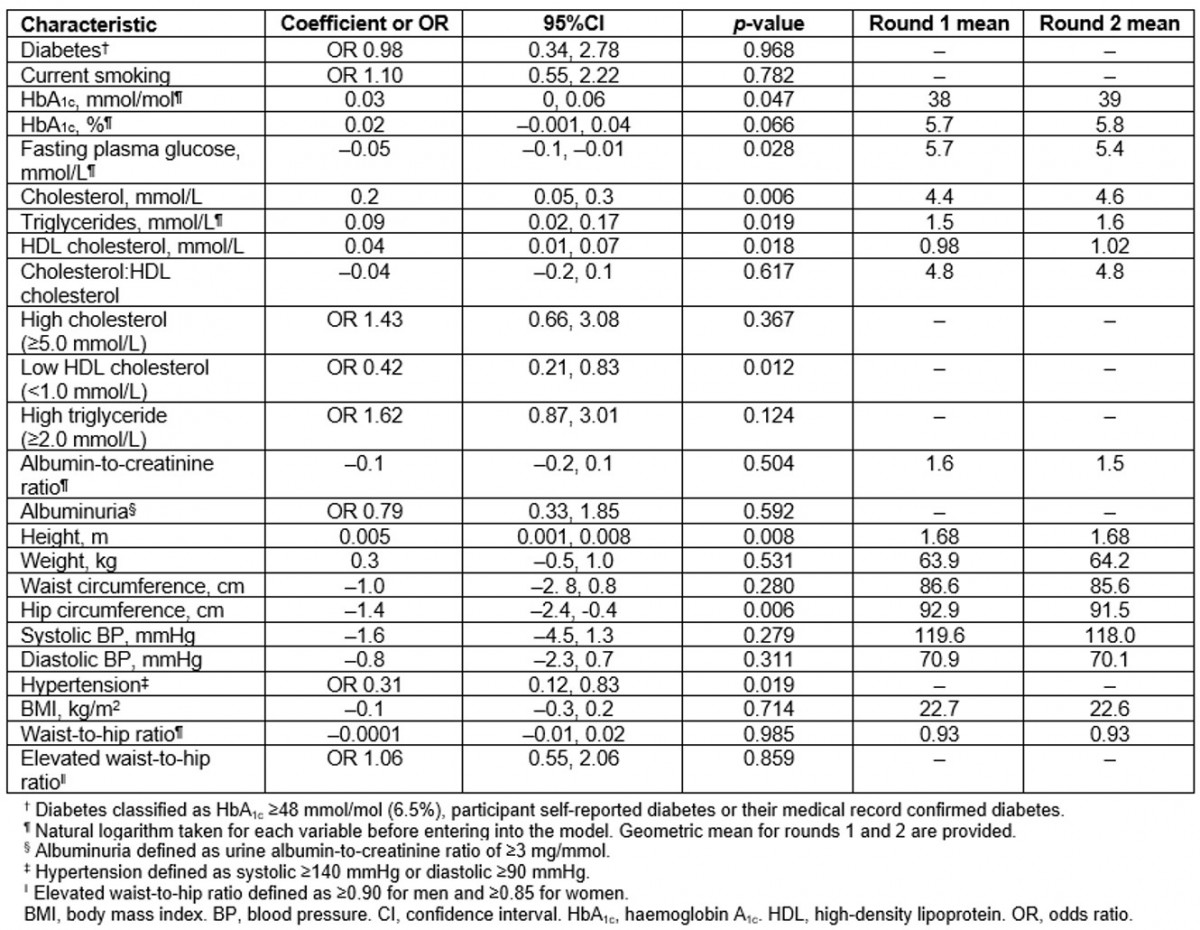
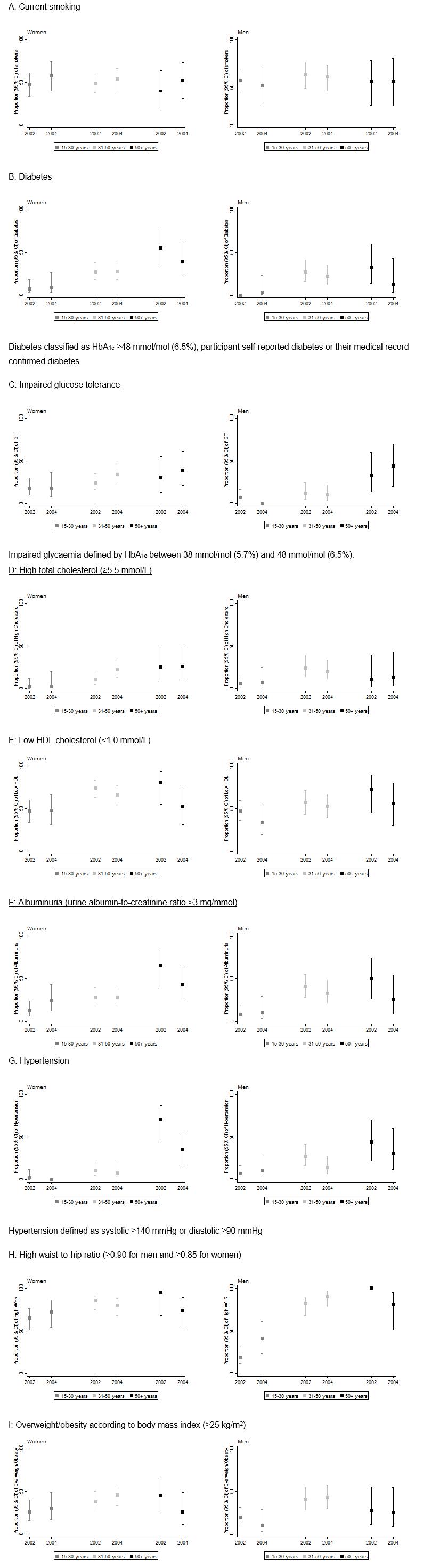 Figure 1: Prevalence of risk factors according to age groups in women and men at first and second surveys.
Figure 1: Prevalence of risk factors according to age groups in women and men at first and second surveys.
Longitudinal analysis
Findings from the longitudinal analysis (n=87) are reported in Table 3. Age- and sex-adjusted means for HbA1c, glucose, cholesterol, ACR, weight, waist circumference, BMI and WHR were higher than the cross-sectional results in both the first and second surveys. However, the change in risk factors over time in the longitudinal analysis reflected the same general trends as observed from the cross-sectional analysis, except for HbA1c, which showed a greater increase across the two surveys.
Table 3: Longitudinal analysis of chronic disease risk factors in participants present at both first and second surveys (n=87) (Data are coefficient for continuous variables and OR for categorical variables. All models adjusted for age and sex)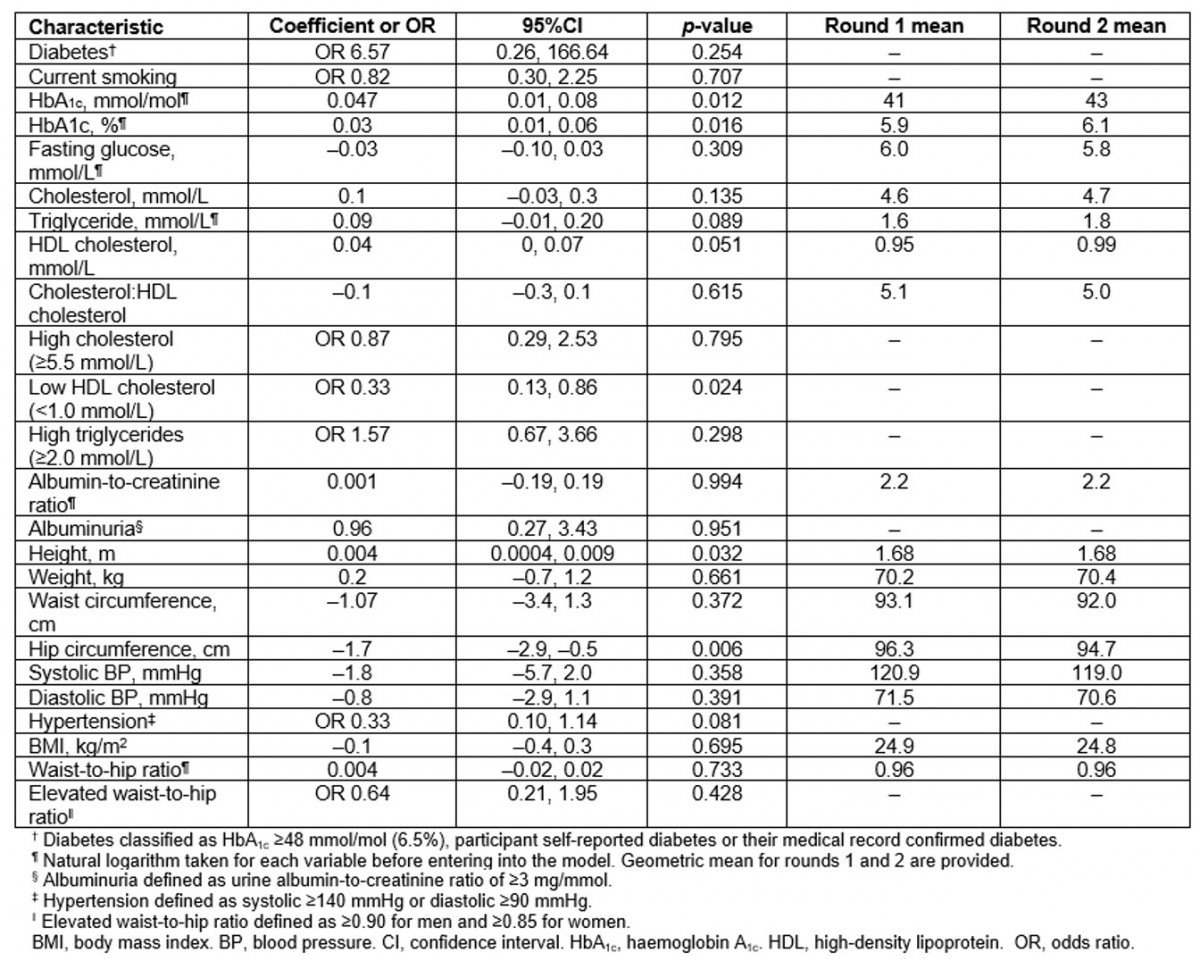
Discussion
This is one of the few studies to report change in the prevalence of chronic condition risk factors over time in an Australian Indigenous community who experienced community-wide health promotion activities over 3 years. The authors observed stable body composition and albuminuria levels, and an increase in HDL cholesterol over time. These findings suggest that the health promotion activities may have had some positive impact. However, given that there was no change in the high smoking prevalence rates, an increase in HbA1c levels for those with diabetes, an increase in the prevalence of impaired glycaemia, and small increases in total cholesterol and triglyceride levels between baseline and follow-up, further efforts to improve health and wellbeing are still required to minimise risk of chronic conditions.
Smoking prevalence in this community was high, but rates were consistent with 2002 national data10. The findings showed no significant changes in smoking rates. This is inconsistent with national data and other literature that report a reduction in smoking rates over time. The Australian Bureau of Statistics has reported a consistent decline in smoking rates in the Indigenous population: from 49% in 2002 to 41% in 2012/1310. A study by Thomas et al also reported a significant decline in national Indigenous smoking prevalence between 1994 and 200831. This equates to less than 1% absolute change in prevalence each year. Similarly, in another Indigenous community, Burgess et al reported a 5% decrease in smoking rates over a 1-year follow up period, following institution of Aboriginal health checks13. The health promotion activities in this community predominantly focused on nutrition and physical activity to align with type 2 diabetes prevention25, and thus further programs targeting smoking cessation are required, and may also need to target young women, given that the authors observed increasing trends in smoking rates for this group.
Diabetes prevalence was high in this remote community and remained stable over the time period. Rates were higher than those reported from national data10, but diabetes prevalence rates are higher in remote than urban Indigenous populations13,19,32,33. The higher mean HbA1c observed in the second survey was largely driven by the higher HbA1c levels among those with diabetes, rather than those without diabetes. These findings indicate that further strategies to improve the management and prevention of diabetes are warranted. Other longitudinal studies of people with diabetes have demonstrated significant reductions in HbA1c after instituting strategies to improve medical management. An 18-month follow-up study of a community-based health worker outreach program reported a 1% decrease in HbA1c34. Furthermore, there was a reported decrease in HbA1c in the urban-based Fremantle Diabetes Study in Western Australia, consistent with improved diabetes treatment14. There are challenges, however, to the successful implementation of chronic disease primary health care for Indigenous people, particularly in remote settings. Strategies that identify and address the enablers and barriers that present to healthcare professionals, patients and their communities are required35.
Overall, the authors observed little change in the prevalence of lipidaemia in this community other than an improvement in HDL cholesterol, which may have resulted from store-based health promotion activities focused on improving fruit and vegetable intake. Whilst prevalence of high total cholesterol was relatively lower than national rates10, rates of dyslipidemia (low HDL cholesterol and high triglycerides) were high. The inertia observed in the prevalence of lipidaemia is surprising given that several of the health promotion activities focused on reducing the consumption of high-fat foods. For example, one program advocated a reduction in the availability of fried foods at the local stores. Other studies of remote communities have reported reductions in total cholesterol levels in the population after the introduction of a community-based nutrition awareness and healthy lifestyle program22,23. However, compared to the activities undertaken in the community in the present study, the programs were undertaken over a longer follow-up period, which may indicate that longer term initiatives are required to achieve change.10,22,23,36,37.
Overall, no increase in the prevalence of overweight or obesity was observed over the follow-up period. This contrasts with findings of an increase in BMI over time24,38. The present findings suggest that the health promotion activities that focused on improving nutrition and physical activity may have had some impact maintaining stable mean BMI and diabetes prevalence in this community. However, the follow-up period was relatively short in this study; longer follow-up is required to ascertain whether the positive impact of these activities persists over time.
Although it is concerning that a number of the other chronic disease risk factors assessed worsened over time, it is difficult to conclude whether or not these results would have been even worse without the health promotion campaigns. This is because there was a not a control group that was not exposed to these preventive initiatives. Potential reasons for the incomplete improvements in chronic condition risk factors in this community may have been attributable to limited sustainable impact in the areas of organisational and community-level policy25. Despite the involvement of multiple agencies in the community-wide health promotion and integration of health-promoting initiatives in service delivery, the impact of community-level change may have been limited by insufficient short-term funding affecting long-term capacity development among community members and growth of governance structures to coordinate agencies to develop policy25. Further, community-led health promotion initiatives alone are unlikely to achieve sustained change in the health and wellbeing of populations. It will be important to simultaneously address social and economic disadvantages experienced by many Indigenous communities. Future efforts to improving structural issues such as education, employment, housing, food affordability and public spaces to promote physical activity are required, in conjunction with policies and programs that address intergenerational grief, mental health and racism4. Consideration needs to be given to the provision of dedicated funding for community-driven and governed health promotion initiatives and supporting policy to further the benefits observed from the health promotion activities that occurred during this project.
The following limitations of this study should be considered when interpreting the findings. First, the analysis was based on around 50% of the community population, and the follow-up survey under-represented younger people and men. This may have affected the generalisability of the study findings to the whole community. Recruitment to studies in remote communities may be hampered by high mobility of the population: people travel between communities and remote centres for cultural and family reasons. Nonetheless, the first survey population was representative of the community population12 and attempts to account for age and gender response bias were undertaken in the statistical analyses. Additionally, repeat analysis in the longitudinal cohort showed similar trends in risk factors over time. Second, the authors were not able to compare the results to a comparison control community not undertaking health promotion activities. Third, smoking was based on self-report and thus subject to recall bias, which may have led to inaccurate prevalence estimates, although high rates reflect rates reported from other Indigenous populations10. Fourth, higher mean values for several risk factors were observed among the longitudinal cohort compared to the cross-sectional analysis, which likely reflected the selection bias of those participants with more chronic disease risk factors being interested in their health and presenting to both surveys. Finally, the data were collected between 2001 and 2004 and may not represent current chronic conditions rates in the community, although the program of work and study processes could still inform future programs. The implementation of electronic medical records data collection in recent years may provide an opportunity to use contemporary data to re-measure rates in this community and provide information on longer term trends.
Further studies on trends in chronic disease risk factors in other communities are required to develop a more complete picture of the changing health status in Indigenous Australians. This may now be possible with the advent of electronic medical records data collection, which will permit assessment of population-level trends in risk factors and conditions over time. Current health promotion activities in Indigenous communities also need to be assessed in terms of their effectiveness from a cultural perspective35. There is limited evidence available to inform policy and practice decisions regarding health promotion initiatives in Indigenous communities, and further research is critical39. The Good Food Planning Tool, developed with the input of this study community, offers a participatory multi-sector continuous improvement approach to support community level decision-making with regards to food security40. Consideration, however, needs to be given to the establishment of dedicated funding of community-based health promotion agencies, whose focus is the development of culturally appropriate, effective and long-term public health initiatives, supported by appropriate government policy to enable the community environment to be a health-promoting one.
Conclusion
This study is an important contribution to the knowledge of chronic disease risk factor trends in a remote Indigenous community on a background of community-wide and community-led health promotion activities. The stability of body composition and increase in HDL cholesterol perhaps reflect the benefits of multisectoral community-wide health promotion initiatives. Nonetheless, the high rates of smoking and dyslipidaemia, the increase in impaired glucose tolerance and increase in HbA1c for those with diabetes over a short period of time require ongoing attention. This analysis provides important quantitative assessment of work undertaken in the community, and it will inform future policy and health promotion initiatives and chronic conditions management that aim to reverse current trends in chronic diseases for Indigenous Australians.
Acknowledgements
The authors are grateful to the Yolngu of north-eastern Arnhem Land for support of this research. Special appreciation is expressed to community researchers of the Yalu’ Marnggithinyaraw Centre, Dorothy Yungirringa and Dorothy Bepuka (deceased) and the Yalu’ clan management committee who facilitated this study and continue to share the findings of this study with their families, community members and other communities in the north-eastern Arnhem region of the Northern Territory, the Community Council, Galiwin’ku, Northern Territory. We also acknowledge the important contributions made by many people who have contributed invaluably to this study: community members and community-based researchers Steven Djati Yunupingu (deceased) and Heather Ganilawur; Cherryl Wirtanen, Joan Djamalaka, David Peiris, Michelle Dowden, Cameron Main and Andrew Knight of Galiwin’ku, Ngalkanbuy Health Service; the diabetes screening planning committee comprising Keith Djiniyini (deceased), Ian Gumbula, Dorothy Wanamula, Stephanie Yikaniwuy and Litia Vuqa (deceased); Federica Barzi (for help with statistics), Geoffrey Angeles, Susan Hutton, Zhiqiang Wang and Stephen Halpin at the Menzies School of Health Research; Shepherdson College; and the Bible Translation Centre.
This work was funded by the National Health and Medical Research Council of Australia (NHMRC) (#124319) and by a grant from the Australian Health Ministers Advisory Council/States-Commonwealth Research Issues Forum (#PDR 2001/07) (MD). ELM Barr was funded by a NHMRC Training Fellowship (#APP1016612) and a National Heart Foundation postdoctoral fellowship (#101291). JB was supported by a National Heart Foundation Future Leader Fellowship (ID: 100085). LMB is supported by an NHMRC Practitioner Fellowship (#1078477). The information and opinions contained in this article are solely the responsibility of the authors and do not necessarily reflect the views or policy of the NHMRC.
References
You might also be interested in:
2009 - Childhood obesity and elevated blood pressure in a rural population of northern Greece


