Introduction
Malaria is a common cause of fever in sub-Saharan Africa (SSA)1 and, until recently, clinical diagnosis based on signs and symptoms was often used to guide treatment decision-making. However, the introduction of malaria rapid diagnostic tests (mRDTs) and new WHO guidelines that recommend testing before treatment have improved providers’ ability to identify those who are infected with malaria2. Importantly, research shows that providers trust mRDT results and are less likely to treat malaria in the absence of a positive mRDT3.
Yet, not all mRDTs support a diagnosis of malaria, challenging providers to determine an alternative diagnosis. A recent literature review of treatment for fever at outpatient clinics in SSA concluded that treatment regimens are often inappropriate and that an understanding of healthcare providers’ practices is necessary for more accurate management of febrile patients4. Inaccurate or delayed diagnosis of febrile illnesses can result in significant morbidity and mortality5. The misdiagnosis of fever results in non-treatment of the actual etiology, which may lead to significant morbidity and death6. Misdiagnosis thus leaves a patient vulnerable to worsening of the underlying illness, and allows for transmission of communicable diseases. Likewise, misdiagnosis can result in inappropriate treatment regimens, such as antibiotics for viral illnesses. In a recent study from rural Tanzania, antibiotics were given unnecessarily 70% of the time when mRDT-negative patients presented with fever and respiratory symptoms7. Misdiagnosis and inappropriate treatment challenges are particularly true in rural areas with limited access to health care, lab infrastructure, or other means of diagnosing the cause of fevers.
Malawi, one of the poorest countries in the world, has areas of high malaria transmission in addition to a high burden of non-malarial fevers from pneumonia, respiratory viruses, and schistosomiasis8. Malawian healthcare providers face challenges similar to those of their counterparts in other areas of SSA; in 2014, almost half of all children with acute respiratory illness who were seen in Malawi government facilities received antibiotics8. Approximately 75% of the Malawian population lives in rural areas with limited access to high-level health care8. Mobile clinics operated by the Global AIDS Interfaith Alliance (GAIA), a non-governmental organization that has worked in Malawi since 2000, help to meet the healthcare needs of rural Malawians. GAIA’s mobile clinics care for 15 000–20 000 rural, underserved patients each month who present with a wide variety of complaints, malaria and fever being among the most frequent9,10. Each GAIA mobile clinic is staffed by a clinical officer, a nurse (or nurse midwifery technician), a nurse’s aide, and a driver. As the GAIA clinics are the primary and often only health facility for much of the rural population they serve, it is critical to understand the etiology and outcomes for mRDT-negative patients, especially given their mobile nature, which may see patients waiting for additional care if the underlying illness is not treated.
Two important problems arise when diagnosis and treatment are incorrect in patients with negative mRDTs: (1) the possibility of worsening disease or death due to misdiagnosis, and (2) the development of antibiotic resistance when antibiotics are prescribed inappropriately. In Malawi, while researchers have explored case management and diagnoses around malarial and suspected-malarial fevers, less is known with regards to non-malarial fevers11,12. The aim of this study is to understand health providers’ practice patterns, both quantitatively and qualitatively, around interpretation of test results, influences on diagnostic and treatment decision-making, and perceived knowledge gaps.
Methods
Study setting and sample
This mixed-methods study was conducted in GAIA mobile clinics in Mulanje and Phalombe districts in southern Malawi. Retrospective data on diagnoses and treatments of febrile illness from seven mobile clinic logbooks were collected for a 2-month period in the dry season (June and July, 2016) and a 2-month period in the wet season (November and December, 2016). All patients presenting at the GAIA clinics presenting with a fever, suspected of one, or having reported a recent one had their temperature taken, and those with a recorded axillary temperature of 37.5ºC or higher received a mRDT (Paracheck Pf, Orchid Biomedical Systems; http:// http://www.tulipgroup.com/Orchid_New/product_range.htm) from a trained nurse or clinical officer. Those with a fever or who had reported a history of fever within 48 hours, and who had a negative mRDT (Paracheck Pf) were included in the analysis. Key informant interviews were conducted with all the mobile clinic health workers, including registered nurses, nurse midwifery technicians, nurse aides, and clinical officers who triage, diagnose, and treat patients as well as dispense medication. Of the 31 eligible mobile clinic health providers, 100% participated in the key informant interviews.
Qualitative data collection and analysis
For the key informant interviews, all health providers on the mobile clinics were approached, and all agreed to participate. Each participant provided written, informed consent (available in both English and Chichewa). Interviews were semi-structured and focused on site- and provider-level factors that influence treatment of patients presenting with a negative mRDT. Questions included content on (1) providers’ training and clinical experience, (2) approach to determining a diagnosis in light of a negative mRDT, (3) availability or use of clinical diagnostic tools, and (4) familiarity with the etiologies and management of non-malarial fevers (Table 1). A trained team of local, independent consultants conducted all interviews. Interviews were conducted in Chichewa, translated, transcribed, and uploaded to a secure database.
Data for the qualitative portion of the study were uploaded to and analyzed in dedoose v7.6.6 (Sociocultural Research Consultants; http://www.dedoose.com). First, using an open coding approach, four interviews were independently coded by two investigators. Any discrepancies were mediated by a third investigator until consensus was reached. Codes were assigned to each construct using quotes that best illustrated the construct of interest.
Table 1: Qualitative constructs and sample questions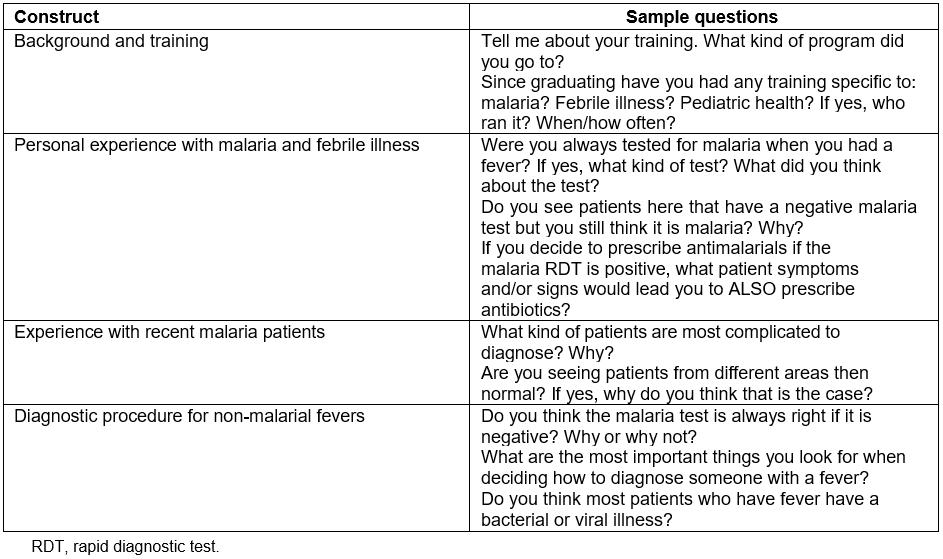
Quantitative data collection and analysis
Data for the quantitative portion of this study were collected retrospectively solely via logbooks from the seven GAIA mobile clinics. All individual-level characteristics were coded and logged into a secure database viewable only by approved study personnel. All personal identifying information, such as name and home location, was removed to protect participant anonymity.
Given the potential for seasonal differences in disease burdens in Malawi, data were collected from both the dry season (June and July) and the wet season (November and December). All patients presenting to a GAIA mobile clinic during these periods and recorded in the logbooks were included for analysis. Demographic characteristics available from clinical records were limited to participant sex, district and village of residence, age, and whether or not individuals had a prior GAIA visit. Records also listed diagnoses and medications, if any, given to patients by the clinical staff. Individual diagnoses were grouped into common, general diagnosis categories for analysis (Table 2).
Descriptive statistics were first calculated to summarize the variables of all patients. Bivariate statistical tests assessed potential differences by season using χ2 tests and Fisher’s exact test when observed cell counts were 0 and/or expected counts were less than 5. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to show both the direction and strength of associations, with a significant association deemed present when the CI did not cross parity. All data analysis was completed using the R statistical package v3.2.4 (R Project for Statistical Computing; https://www.r-project.org).
Table 2: All diagnoses from malaria rapid diagnostic test patient records from retrospective logbooks, June–July 2016 (dry season) and November–December 2016 (wet season) 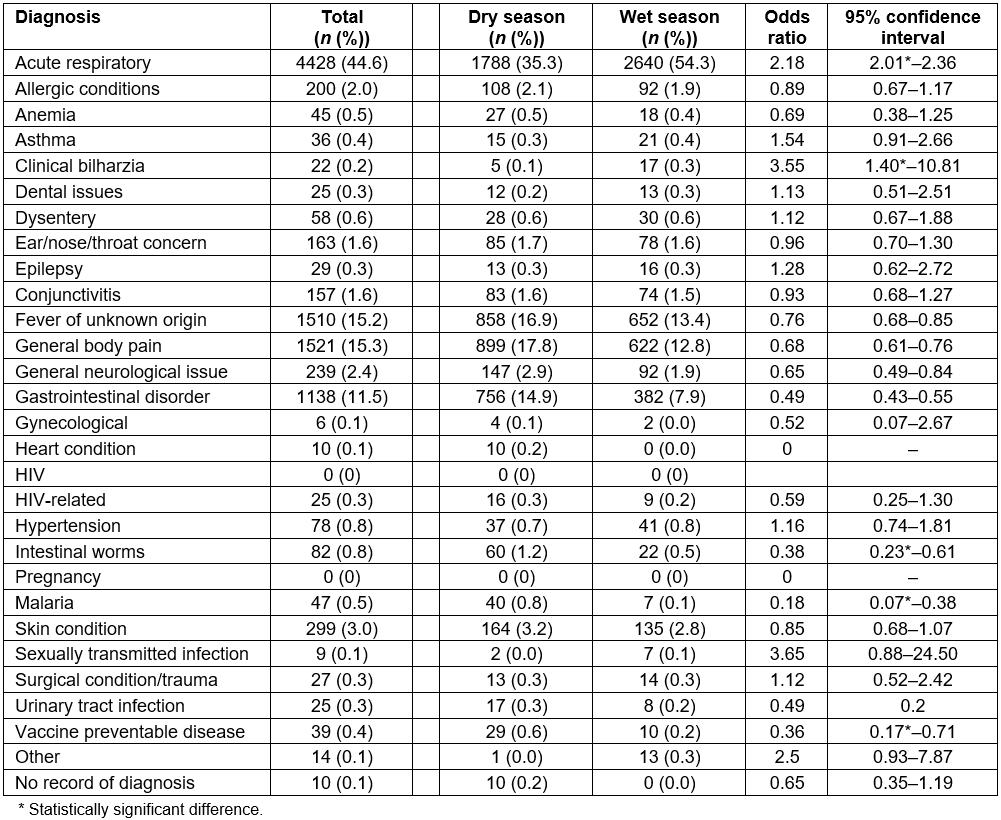
Ethics approval
This study was approved by the University of California San Francisco Human Research Protection Program (approval #16-19187) and the Malawi National Health Sciences Research Committee (Protocol #16/5/1594).
Results
In total, 30 672 febrile patients were seen during the study period. Of those, 9924 (32%) tested negative for malaria by mRDT. Clinical records of all mRDT-negative patients were reviewed retrospectively to ascertain final diagnosis, and treatment prescribed in both the wet and dry season. Next, interviews were conducted in August-–September 2016 with 31 key informants (Table 3). The interviews explored past clinical training, healthcare providers’ perceptions affecting diagnosis and management of febrile illnesses, providers’ approach to the diagnosis and treatment of febrile illnesses, as well as knowledge surrounding the etiology of non-malarial fevers.
Table 3: Key informants from Global AIDS Interfaith Alliance mobile clinics
Quantitative results
Out of the 9924 mRDT-negative patient records examined over the 4-month period, 6004 (60.5%) were female compared to 3881 (39.1%) males (Table 4). There was no significant difference in the number of female versus male patients in the dry and wet seasons. The majority of patients were from Mulanje district (87.7%), with a smaller percentage coming from Phalombe district (12.3%). Approximately 32 days (27%) of logbook data from the Phalombe district were unavailable for review during the wet season analysis. To assess whether this would bias the subsequent analyses, a full comparative analysis was run comparing Phalombe patient characteristics to the rest of GAIA patents overall and by season. No significant differences were found. First-time patients were significantly more likely to visit mobile clinics in the wet season compared to the dry season (OR 1.56, 95%CI=1.41–1.75).
The official recording of multiple diagnoses was quite rare, with 96.0% of patients receiving only one, 3.5% receiving two, 0.4% receiving no official diagnosis, and the remaining <0.1% (n=3) having three or more (Table 5). Acute respiratory infection was the most common diagnosis for mRDT-negative patients (44.6%), and this number increased in the rainy season as compared to the dry season (54.3% v 35.3%, respectively, OR=2.18, 95%CI=2.01–2.36). The second most common diagnosis was general body pain (15.3%), with significantly fewer cases diagnosed in the wet season compared to the dry season (12.8% v 17.8%, OR=0.68, 95%CI=0.61–0.76), followed by fever of unknown origin (15.2%), also seen less frequently in the wet season compared to the dry season (13.4% v 16.9%, OR=0.76, 95%CI=0.68–0.85). The only additional diagnostic category representing greater than 10% of all diagnoses was gastrointestinal disease (11.5%), with a significantly lower number of cases seen in the wet season when compared to the dry season (7.9% v 14.9%, OR=0.49, 95%CI=0.43–0.55). Table 2 shows all diagnoses given during the study period.
Of the total patient records reviewed with a negative mRDT, only 59 (0.6%) patients were prescribed antimalarial drugs. Over half (60%) of mRDT-negative patients received antibiotics as a treatment. Antibiotics were prescribed primarily for acute respiratory illness (54% of all patients), followed by fever of unknown origin (25%; Table 6). Pain relief (acetaminophen, non-steroidal, aspirin) was given to 81.1% of patients, but less often in the wet season (OR=0.65, 95%CI=0.59–0.72). With regards to overall prescribing behavior by GAIA staff, slightly over one-third (35.1%) of patients received a single medication, over half (57.8%) received at least two courses of medication, and, at most, patients received four medications, occurring for 26 (0.3%) patients. Only 27 patients out of 9924 did not receive any medication at all.
Table 4: Summary of malaria rapid diagnostic test negative patient demographic variables, by season 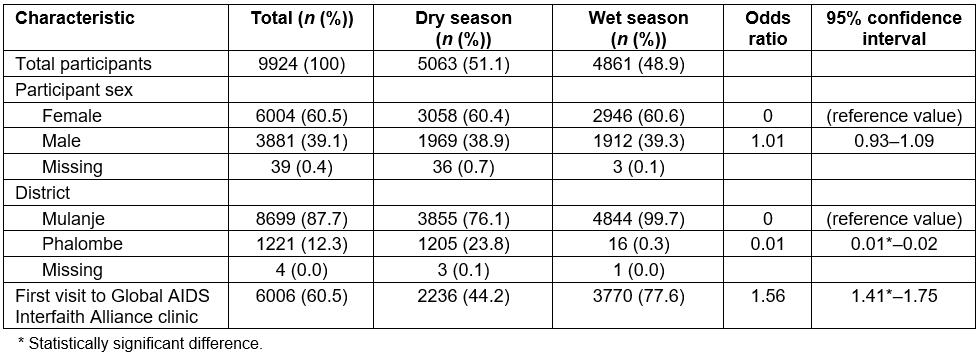
Table 5: Total number of recorded diagnoses for malaria rapid diagnostic test negative patients in logbooks, June–July 2016 and November–December 2016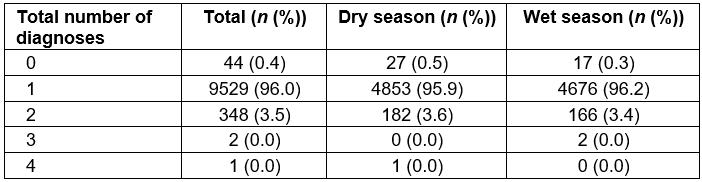
Table 6: Diagnosis and corresponding medication prescribed for malaria rapid diagnostic test negative patients from retrospective logbooks for five most frequent diagnostic categories, June–July 2016 and November–December 2016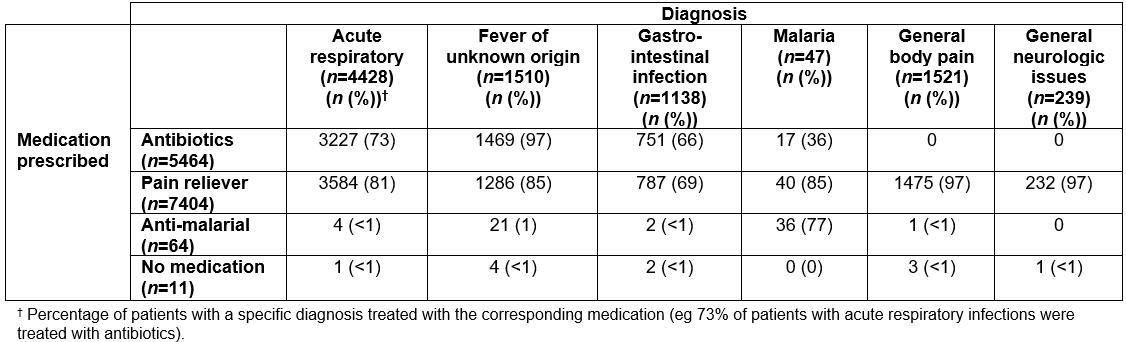
Qualitative results
Four key themes were identified during the analysis: (1) background and experience of health providers, (2) health providers’ recent experience with malaria patients, (3) health providers’ personal experience with malaria and fevers, and (4) how providers treat non-malarial fevers.
Background and training: The range of experience among the health providers on the mobile clinics was expansive. Seven clinical officers were interviewed, with experience ranging from 7 to 35 years. Nurses interviewed included student nurses, nurses’ aides, and nurse midwifery technicians. Training in pediatrics was limited, with most health providers stating they learned on the job.
We learnt this from our classes for 2 weeks [and] after that we did our practices, [which included] almost 3 weeks managing malnutrition [sic] children at rehabilitation. [H]onestly, I have not yet trained [in] this after graduating, I learnt this at school. (Registered nurse)
We were taught about triage … we were trained to pick children who are seriously ill and put them first on the line and how to check temperature.
[Who did this training and for how long?]
They just briefed us here at GAIA, our colleagues. (Nurse midwifery technician)
However, six of the seven clinical officers had undergone additional training that focused specifically on malaria since 2013. All of the clinic nurses had malaria coursework in the past 3 years. The one rotating nurse, educated as a clinical officer, conducted classes training providers in malaria management. Only two of the follow-up coordinators had recent malaria training, while none of the nurse aides had been trained via a malaria course. The trainings were offered by GAIA (2014, 2015), the Ministry of Health (2013, 2015, 2016), and the district health officer (2013).
Only 7 of the 31 mobile clinic staff reported having had specific training in or around the subject of febrile illnesses, yet most respondents noted that these courses were many years ago, and lacked specifics as to what was covered. Additionally, none mentioned a course specifically on fever diagnosis or management itself. Several staff mentioned that they learned about fever and aspects of febrile illness treatment in the context of other courses (eg Integrated Management of Childhood Illness (IMCI)). Significantly, 24 of the mobile clinic staff stated they had not had any additional training around febrile illnesses as a stand-alone topic, especially with regard to mRDT-negative patients.
[What about training on febrile illness?]
Yes, I have had training on that too.
[What did you learn?]
Ummh … (hahahaha) … it’s been a long time. (Nurse midwifery technician)
[Have you had any training specific to febrile illness?]
I haven’t been trained since graduating. (Nurse midwifery technician)
Recent experience with malaria patients: All staff knew that patients with a positive mRDT needed treatment for malaria, and that the drug of choice on the mobile clinics was lumefantrine/artemether (LA). However, there was some disagreement and/or confusion when asked under what circumstances an mRDT-positive patient would not receive an antimalarial. Interviewers asked providers about patients who had recently been given LA (eg in the past week) but were still symptomatic with a positive mRDT and would not be appropriate candidates for re-treatment, either because they were still taking LA or there were concerns about possible drug resistance: these patients would be referred.
Very few cases are treated for malaria though the result was negative. A person who has been on LA treatment and 2 weeks has not yet elapsed but is still presenting with signs of malaria, we refer [that] person to be tested using microscopy. If the microscopy finds the parasites within the 14 days then the patient is given a second line treatment [for] malaria. (Clinical officer)
While a few staff thought that patients were taking their medications (based on their observation that the patients did not return to the mobile clinic, thus assuming they had recovered), more were of the opinion that patients were not adherent and were not completing the course of medication. A number of issues were identified as contributing to medication non-adherence: one family member would come to the clinic and obtain medication, but then share it with other family members who were also ill; after a few doses, the patient felt better so stopped taking the remaining medication (saving some for next time); and limited literacy precludes taking the medication as prescribed (eg right number of pills per day, correct number of days of treatment).
We have a very huge problem. It seems like most of the people share with friends the medications that we prescribe to them. [U]nfortunately they do not come to us to report that they have shared the medication with friends [and], in the end, they take inadequate medication. (Clinical officer)
Personal experience with malaria and febrile illnesses: Almost without exception, the mobile clinic staff had personally experienced malaria, and thus were very familiar with the symptoms and treatment. Some reported having had malaria only as a child, while others have had malaria repeatedly and recently, including within the previous few months.
The majority experienced testing with mRDTs; some had microscopic testing. Several relayed that they had not had malaria since they began using bednets consistently. Some staff reported that they had tested negative by mRDT, but subsequently were positive for malaria parasites on microscopy.
A number of those interviewed reported being treated with antimalarials despite having a negative test, or no test at all.
Whenever I get tested for malaria, the results come out negative. Even though this is the case, I just take [an] antimalarial and after finishing the dose, I get better. (Nurse midwifery technician)
When I have the … signs firstly I do take antibiotics to my surprise I do not get better. Once I take LA the situation improves so I am not sure whether it is really malaria or infection. (Clinical officer)
Diagnostic and treatment procedures for non-malarial fevers: Antibiotics were frequently prescribed when the mRDT was negative, as well as regardless of the mRDT result, especially if there was a cough, high fever, or diarrhea. Answers regarding antibiotic prescribing patterns were similar regardless of the training level or role of the respondent. A frequent misconception among providers was how a viral infection is treated; many thought antibiotics could be given for viral infections. The different antibiotics (amoxicillin, erythromycin, sulfamethoxazole/trimethoprim, penicillin, metronidazole, doxycycline, gentamicin) were used interchangeably and prescribed based on drug availability as opposed to clinical presentation.
[C]linically it becomes very difficult to be sure that you are really treating for malaria so what you do is, you prescribe medication to cover maybe for malaria and for a bacterial infection. (Registered nurse)
[Y]ou have tested someone with malaria and at the same time you find out that the same person is coughing, so definitely you can’t just treat that person for malaria and leaving the person without antibiotics, that’s wrong. (Registered nurse)
In general, providers felt that they did not have enough efficient, reliable equipment or resources to diagnose patients accurately and often referred patients in such cases.
In brief, this is a mobile clinic, we don’t actually have all the equipment to use in our clinics apart from the professionalism of our clinic personnel and just a few resources we have which are not … comprehensive to give a clear diagnosis. (Registered nurse)
Further, children, the elderly, patients with viral infections, and those who were HIV positive were reported as challenging to diagnose due to difficulty obtaining a history and a lack of diagnostic tools to aid in the differential diagnosis.
Children under five are difficult to diagnose because it’s the mother or guardians who tell you the symptoms and signs and not the child, so sometimes they may exaggerate or not say enough for proper diagnosis to be made. (Clinical officer)
Elderly people are difficult to diagnose because they forget to tell you other complaints that they have that can help you make the right diagnosis, they just tell you one or two and you really need to probe much to get to the bottom. (Nurse midwifery technician)
Almost all providers referred to the WHO IMCI approach, Malawi Clinical Guidelines, and Malawi Standard Treatment Guidelines, though there was some variability regarding which guidelines were most influential. Multiple providers mentioned that they would like additional or refresher training for key guidelines, such as IMCI. One provider expressed frustration with the malaria guidelines, specifically, how to manage an mRDT-negative patient with signs and symptoms of malaria.
[H]onestly, we don’t have the guideline … [W]e have requested for them before … to give us guidelines, treatment guidelines. We have STI [sexually transmitted infection] treatment guidelines, we have malaria treatment guideline … So I only use my knowledge that I got from the training on malaria treatment guidelines. But apart from that, we don’t have written document or hard copy of guidelines that we can follow … I think trainings are very important for us, because they keep us updated. (Clinical officer)
The guidelines are silent; they left as with a gap. There is no option of either giving the patient antibiotics or antimalarial. I have read the book for several times. I have searched, but still today, I have not found the information on how to manage a patient with fever, typical signs of malaria and [a] negative result for [a] malarial test. (Clinical officer)
In general, providers repeatedly mentioned that the following conditions should be referred: danger signs (high temperature, fainting and weakness), malaria treatment failure, need for oxygen, need for intravenous medications, convulsions, dehydration, lethargy, anemia/pallor, loss of consciousness, and shortness of breath. Several providers mentioned the Emergency Triage Assessment and Treatment (ETAT) guidelines as a system for triage. Others mentioned the reluctance of patients, and especially parents, to agree to referral due to unpreparedness. Still other providers were concerned about patients dying ‘in the process of referring them to the hospital’ due to a lack of supplies and personnel.
The other challenge is when you refer the child to the nearest health center the hospital would say, ‘We don’t have the drugs’. You go as far as (another town), they tell you the same story, ‘We don’t have the medication, just keep going’, until our drivers come back here a distance of about 60km from our center … So when the medicines are unavailable in the hospitals, it scares us a lot. (Clinical officer)
There were many misconceptions regarding what presents with a fever, including hypertension, epilepsy, sexually transmitted infections, urticaria, conjunctivitis, or pregnancy.
[Apart from malaria, what are the other common causes of fever you see here …?]
Aaah, maybe blood pressure, sometimes it causes fever, epilepsy and cough also cause fever. (Nurse aide, high school certificate)
It happens that you don’t have malaria so it can be other disease. So we give them antibiotic because they treat viral infections. Some fevers are caused by virus. (Nurse midwifery technician)
Some providers thought that the majority of non-malarial fevers were pneumonia. Other providers appropriately used the history, patient symptoms, the physical exam, and diagnostic test results to build a differential and rule in or out specific diagnoses.
I think the first thing we would test child. If the test is negative, upon examining that is … you can also check the chest, like listen to the chest, the breathings how the respiratory is putting up, the heart. If you hear something like crackles, you would say maybe it’s pneumonia and then you treat as pneumonia, you give an antibiotic. (Clinical officer)
Discussion
With declining malaria transmission in many SSA countries and the introduction of mRDTs to rule out malaria as a cause of fevers, understanding how to manage non-malarial fevers is increasingly important. Simultaneously, the rise in antimicrobial resistance globally makes reducing the overuse of antimicrobials, while identifying those who need them, critical. Almost all the health providers in this study reported limited training in non-malarial fever management, despite the fact that roughly 30% of all patients with fever seen at the mobile clinics tested negative by mRDT. Furthermore, 60% of patients seen during the study period who tested negative for malaria were given antibiotics.
This practice of providing antibiotics when there is a negative mRDT is consistent with other published studies. In a recent study in Malawi, 59% of patients received antibiotics without an indication and children with negative mRDTs were 17 times more likely to receive antibiotic overtreatment than mRDT-positive children13.
Providers in the present study were influenced in their diagnostic and treatment decision-making by everything from a personal history of malaria, to patient clinical presentation, to personal responsibility should a patient deteriorate before the next mobile clinic visit. First, without additional tools to diagnose fevers, providers in this study relied heavily on patient history to guide their decision-making. Further, mobile clinics visit the study areas once every 7 days, so providers were reluctant to withhold antibiotics for mRDT-negative patients in the event that the patient worsened before the next clinic visit. This may be due in part to the fact that acute respiratory illness was the most common diagnosis in this study population (after malaria), and findings in similar settings have shown pneumonia as the largest infectious killer of children14. Similarly, a recent study in Malawi found that children diagnosed with pneumonia based on IMCI guidelines of observing fast breathing who did not receive antibiotics had worse outcomes when compared to children who did receive antibiotics15. However, withholding antibiotics has its merit, for while the incidence rate for acute lower respiratory infection in Malawi, which includes bacterial pneumonia, is 24% in children under age 5 years, of the children with an identified cause of acute lower respiratory infection, 84.6% were caused by a viral infection, against which antibiotics are completely ineffective16. One of the primary challenges in this scenario is the difficulty in accurately counting fast breathing, a key assessment for pneumonia in settings without sophisticated diagnostic tools, which could be largely addressed by additional simple point-of-care tests, including pulse oximeters17.
The general belief in and adherence to the mRDT test results by GAIA providers is important to note as they only diagnosed and treated malaria clinically in 59 of the mRDT-negative cases during the dry season. This overall 0.6% discordance is in contrast to many other settings and stands out, as a recent systematic review found that making treatment decisions based on mRDT results was most common in areas where the true prevalence of malaria is low, which is not the case in Malawi18. It is possible that providers in the present study used their personal experience of having a negative mRDT but positive microscopy for malaria to justify a clinical diagnosis of malaria. Several providers interviewed in this study considered the presence of other human malaria infections as causing fevers, given the current mRDT used is Paracheck Pf, which tests for Plasmodium falciparum only. Further, many providers interviewed in this study perceived co-infection to be possible in many cases. Several studies cite significant rates of bacterial co-infection with malaria19-22; however, studies in Ghana and Malawi found bacterial co-infection rare among patients with P. falciparum23,24.
Providers in this study had a wide range of educational backgrounds. There was a general misunderstanding of how to treat viral versus bacterial illness, especially among those with fewer years of formal healthcare training. Several providers mentioned hypertension, sexually transmitted infections, menstrual cycles, and epilepsy as causes of fever. These misconceptions lead to additional confusion around how to treat fevers. Further, inconsistent use of guidelines resulted in varying degrees of healthcare delivery among the mobile clinics. Despite the high volume of pediatric patients seen at the mobile clinics (79% of patients during the study period), only three of the providers interviewed had had access to general pediatric training since completing school. One provider last studied pediatric management in 1994. Malawi has made steps to address such concerns by setting guidelines for standardized trainings pertinent to specific areas of expertise (eg ETAT for triage, IMCI, and Malawi Standard Treatment Guidelines)25-27, which were noted as having been taken by some of the clinical participants. A more widespread uptake of these trainings, and expanded requirement of them within specific fields, may help improve overall confidence in diagnostic and treatment decisions. Recent updates on management of common childhood illnesses are available from the WHO for settings such as this and may be useful to incorporate into regular in-service sessions26. In particular, ETAT training may be useful to help guide who and when to refer patients to higher levels of care28.
In general, providers in this study felt that they did not have the appropriate diagnostic tools and resources to diagnose patients accurately. This may help explain the high levels of prescriptions given by GAIA staff, with only 0.003% of patients receiving no medication during their visit, and over half receiving more than one medication. There are a number of low-cost, low-maintenance diagnostic tests currently available for use in such settings that can help diagnose not only disease overall but also disease severity. It is possible that portable, battery-powered pulse oximeters29, point-of-care C-reactive protein22, urine dipsticks to perform rapid urinalysis, and point-of-care rapid hemoglobin tests may strengthen the mobile clinic providers’ ability to identify patients needing antimicrobial treatment or referral to a higher level of care. Indeed, studies from similar settings have also highlighted the need for such tests, especially around non-malarial fevers30,31. Perhaps more importantly, these relatively low-cost additions to the mobile clinics and in similar settings do a great deal towards ensuring that patients, regardless of their status, residence, or ability to pay, have the same access to quality care as anyone else.
Limitations
The mixed-methods approach used in this study sees it facing some of the limitations inherent in each methodology on its own. Quantitatively, despite using data from both the wet and dry seasons, it is possible that diagnoses and treatment from other times of the year would see differing results. The use of the Paracheck Pf mRDT focuses on Plasmodium falciparum and therefore other malarial infections may have been missed. Qualitatively, the results are subject to both response and recall bias, as potentially seen in the affirmative responses to education around febrile illness training but a lack of additional information on it. The researchers sought to address this by confirming participant narratives with their known education backgrounds and guidelines. Overall, the confirmation of findings for both the qualitative and qualitative components lend strength towards the reported findings.
Conclusions
In conclusion, health providers in this setting have limited tools to employ with febrile mRDT-negative patients. Without further diagnostics, practice patterns will likely continue that risk over-prescribing antibiotics due to the difficulty of discerning viral from bacterial infections and providers’ concern that patients will not have access to follow-up care should their condition worsen. While standard guidelines exist for low-resource settings, limitations for mRDT-negative patients and barriers to consistent use warrant further investigation.
Acknowledgements
The authors thank the district health officers in Mulanje and Phalombe districts for their support, as well as the University of California San Francisco School of Nursing and Resource Allocation Program. They also thank Joyce Jere, country director for GAIA, and the health providers and patients from the GAIA mobile clinics for providing valuable insights and access for this work.
