Introduction
A full blood count (FBC) is one of the most frequently ordered blood tests in both primary and secondary care, and most medical emergency guidelines include an FBC as part of the initial clinical assessment1,2.
Although the value of point-of-care (POC) biochemistry assays in the rural and remote setting is well established3-5 the development of POC haematology analysers has been slower, and devices suitable for use in the rural context have only recently become available2.
No previous published studies or evaluations regarding the clinical use of a POC haematology FBC analyser in the rural acute care setting were found. A white cell count POC device improved decision making in routine medical care in a remote-from-laboratory setting in Australia6. An evaluation of a POC haematology FBC analyser in a laboratory research setting concluded that the device would be suitable for use in a small laboratory or as a back-up analyser7.
Hokianga Hospital in the far north of New Zealand (NZ) is a small rural-community-owned and governed hospital which forms part of an integrated health service8. Hokianga, although historically and culturally rich, is today socioeconomically disadvantaged9,10. The population is around 6500, predominantly Maori (70%) and dispersed over a geographical area of 1520 km2 on the rugged north-western coast of Northland, NZ8. The Hokianga Health Enterprise Trust is contracted by the regional (Northland) District Health Board (NDHB) to provide its hospital service. Hokianga Health is acknowledged by the NZ Ministry of Health and NDHB as a Maori Health provider8. The Trust’s health services are distinguished by the kaupapa (policy or purpose, NZ Maori) of its model of care which is uniquely Hokianga, Maori and community focused and includes no payment for service at the point of need and integrated care from community to clinic to hospital8.
While it is well known that Maori are disadvantaged in health outcomes and access to health services, Maori living rurally may face even greater disadvantages than their urban counterparts11.
Hokianga Hospital has ten acute beds and provides in-patient and emergency 24-hour care. Salaried rural generalists provide all local medical services12. Base hospital services are provided 130 km away at Whangarei Hospital and the nearest tertiary referral centre (providing services such as interventional cardiology, neurosurgery and vascular surgery) is 280 km away in Auckland.
There is no onsite laboratory service in Hokianga. A laboratory service fully funded by the NDHB is available from Monday to Friday; specimens are dispatched once daily by courier road service to Whangarei.
In 2008 a hand-held POC analyser providing blood gas, lactate and biochemistry tests was installed at Hokianga Hospital. The associated quality assurance (QA) program was provided (in accordance with national best practice guidelines for POC testing13) in partnership with the NDHB laboratory at Whangarei Hospital. The availability of POC blood tests resulted in improved acute care and an overall reduction in healthcare costs, helping to address prior inequities in laboratory access for the Hokianga population5. POC testing had not been part of the traditional NDHB laboratory funded service thus the costs of POC fell largely to the local health trust.
In February 2016 a POC haematology benchtop analyser (Abbot CELL-DYN Emerald 18 analyser Abbott Diagnostics, Auckland, NZ) was installed at Hokianga Hospital. This analyser reported an FBC with red cell indices, a three-part white blood cell differential and a platelet count. A QA program guided by the analyser’s supplier, supported by the NDHB POC-testing coordinator and delivered at a local level by Hokianga Health registered nursing staff, was implemented.
Before the analyser’s installation, the turnaround-time for an FBC at Hokianga was a minimum of 8 hours on weekdays and up to 72 hours or more over weekends and public holidays.
The aim of this article is to describe the impact of the introduction of POC haematology at Hokianga Hospital on patient care, the acceptability to medical and nursing staff and a cost–benefit analysis. The evaluation of the QA program, including the experience of implementation from a local staff perspective, is described elsewhere14.
Methods
This was a mixed methods study combining quantitative and qualitative components and a cost–benefit analysis using an integrative process: quantitative and qualitative data were analysed concurrently but separately, and integrated during interpretation as previously described15. The study was conducted at Hokianga Hospital.
Part I: Prospective observational study
The analyser was installed in February 2016. Data were collected from February to April and again from June to August 2016. Twelve doctors in total were working at Hokianga Hospital during the study periods, including three short-term locums and one registrar in the first time period. All agreed to participate. Each time a POC haematology test was performed the doctor completed a form, adapted from a previous study16. The form included questions about differential diagnoses and patient disposition before and after the test. Disposition was coded as ‘transferred’ (to base hospital), ‘admitted’ (to Hokianga Hospital) or ‘discharged home’. Changes between the pre- and post-test differential and planned disposition were used to determine the impact of the test on patient management. Data from completed forms was transferred onto a spreadsheet. Statistical analysis was undertaken using the Statistical Package for the Social Sciences v23 (IBM; http://www.spss.com). Descriptive statistics were used to describe study outcomes. Chi squared test was used to test the difference between pre-test and post-test disposition and number of differential diagnoses.
Part II: Semi-structured interviews
Three focus group interviews and one individual interview were conducted between June 2016 and January 2017. All doctors, nurses and Taumata (Hokianga Health cultural advisors) working at Hokianga Hospital at the time of the study were invited to participate. The interview moderators were two of the research team (KB and CB) both also clinicians at Hokianga.
The interview schedule was semi-structured and included questions about how the introduction of the POC haematology analyser had affected day-to-day practice and patient care and questions about technical aspects of set-up and maintenance of the machine.
Interviews were between 15 and 25 minutes in length and were digitally recorded and transcribed.
The transcripts were uploaded to N Vivo v11 (QSR International; https://www.qsrinternational.com/nvivo/nvivo-products). Data underwent an iterative thematic analysis process occurring over several months, with frequent review of the coded data and the emergent themes. While some themes were predefined by the interview questions the analysis also incorporated unanticipated findings. Participant responses were reported as summaries and quotes. To maintain participant anonymity, all respondents were designated a number (1–17) and were referred to throughout by this coding (e.g. respondent 5 or R5).
Part III: Cost–benefit/financial analysis
The costs and benefits associated with the introduction of the POC haematology analyser and its associated QA program were analysed. Tangible costs included the cost of the analyser and other consumables, staff and training costs (based on measured nursing time) and overheads. Tangible benefits relate to savings derived from changes in patient disposition (admission, transfer or discharge).
Ethics approval
Ethics approval was granted by the NZ Northern B Health and Disability Ethics Committee in January 2016 (16/NTB/2).
Results
Part I: Prospective observational study
Over the study period 116 POC haematology test samples were undertaken. Nineteen results were excluded, 16 because the data included on the form was incomplete and three because the test was performed without the doctor having requested them and were not clinically indicated. The remaining 97 tests were included in the study.
On average six POC haematology tests were performed each week. Almost all tests (97%) were undertaken in the setting of the acute undifferentiated patient presentation. Seventy two percent of the tests were performed out of hours (weekends, evenings and overnight). Seventy-one percent of the tests carried out were for patients of Maori ethnicity.
Differential diagnosis: Changes in the number of differential diagnoses before (pre) and after (post) the test are illustrated in Figure 1. The average number of differential diagnoses reduced from 2.43 (pre-test) to 1.7 (post-test) (χ2 tests p<0.05). In 42 (43%) cases, differentials dropped by one. For a small number of cases (3%) having access to the test increased, rather than reduced, the number of differentials.
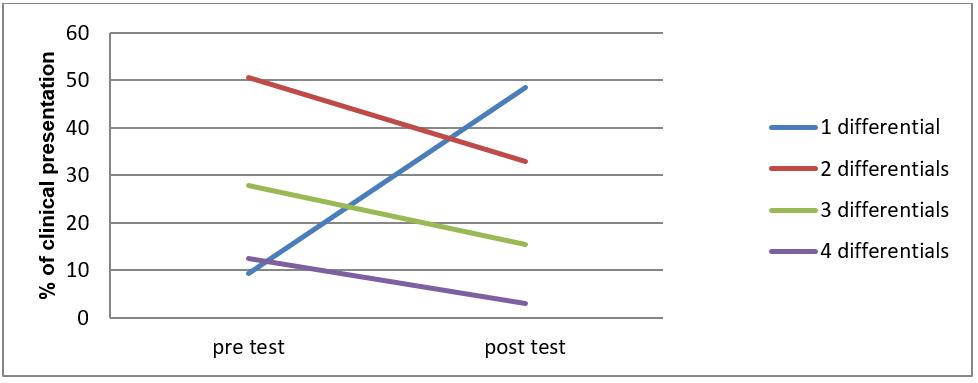 Figure 1: Changes in the number of differential diagnoses before (pre) and after (post) the point-of-care haematology test.
Figure 1: Changes in the number of differential diagnoses before (pre) and after (post) the point-of-care haematology test.
Disposition: There was a significant reduction in the number of patients transferred and an increase in the number of patients discharged home (χ2 tests p<0.05). Prior to the test, 25 (26%) patients would have been transferred to Whangarei Hospital. Post-test this number was reduced to 11 (11%). Of the 59 (61%) patients who, pre-test, would have been admitted to Hokianga Hospital, post-test 14 (14%) were discharged home and 4 (4%) were transferred. All 13 (13%) patients who were expected pre-test to be discharged, were discharged post-test. Figure 2 illustrates the patients’ disposition pre-test and post-test.
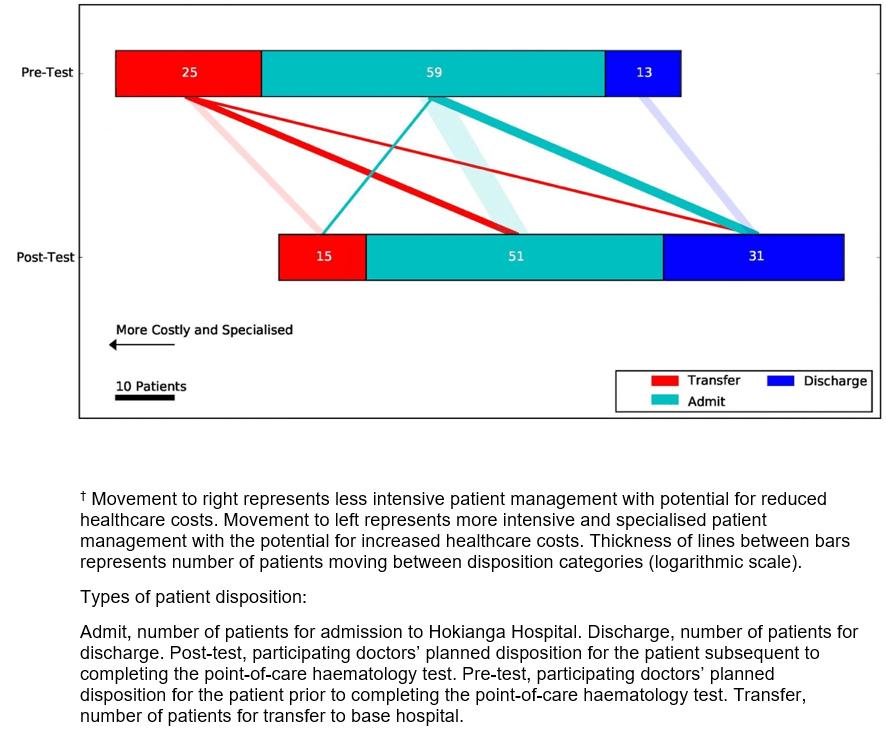 Figure 2: Effect of point-of-care haematology results on doctor’s intention to admit, discharge or transfer a patient to a base hospital.†
Figure 2: Effect of point-of-care haematology results on doctor’s intention to admit, discharge or transfer a patient to a base hospital.†
Change in treatment: Clinicians reported that in the majority of cases (62%) the test altered clinical management. For nine (9%) cases, the change in management was ‘significant’. Examples of significant change included confirming neutropenia in a febrile patient undergoing chemotherapy, excluding significant blood loss in suspected acute gastrointestinal haemorrhage and confirming severe anaemia requiring transfusion. ‘Some’ change predominantly related to a change in the management plan or a reduction in the number of differentials, for example assisting the decision not to start antibiotics.
Part II: Interviews
All eight doctors employed at Hokianga Hospital at the time of the interviews agreed to participate. Seven of these eight participated in the first interview (one was on leave) and four of the eight in the second (four were either on leave or had a roster clash). Of sixteen nurses who worked in the hospital, eight participated in the second focus interview. All agreed to participate but eight were unable to attend due to leave or a roster clash. One individual interview was conducted with a member of the Hokianga Health Taumata who also participated in one of the focus interviews.
Three main themes were identified: impact on patient management, challenges and commitment.
Impact on patient management: Having an FBC result available had a positive impact on patient management mainly by increasing diagnostic certainty, leading to improved clinician confidence:
… the differential diagnosis was septic arthritis versus gout. I know you can get a raised WCC in gout – it just helped us swing it. We’re well aware of the fact that clinically speaking you can have anybody with anything with a completely neither low nor high white count, but they [an FBC result] push you in one direction or another. R1
… any little bit of back up that supports what you initially thought clinically it all helps [everyone nods in agreement]. R7
An FBC result informed and facilitated discussions regarding management including disposition , both with patients and with base hospital specialists:
… the patient with the haematological diagnosis, I was able to bring her in here, confirm the results and still ended up sending her on to Whangarei, but being able to actually confirm that result and say: okay yeah this is really what it is, made it much easier for her and her husband to understand why they needed to head down [to Whangarei Hospital]. R3
Following acute GI [gastrointestinal] bleeds … when people appear to be stable, it does actually enable you to confirm that, without them having to either go walloping off to emergency, or having that sort of uncomfortable delay, for example over a weekend. R1
Challenges: These related predominantly to the indirect costs of implementing a benchtop analyser and its associated QA assurance program in a setting with no onsite laboratory. Operating the analyser required substantially more resource than the hand-held device installed at Hokianga Hospital 8 years earlier. Meeting the technical aspects of the day-to-day running of the machine fell largely on the Hokianga nursing team, on top of their already full workload:
Sometimes it’s been a pain, because it’s not working, something’s full and needs emptying at an inopportune time or the cleaning fluid or the lyse (lyse solution) or something like that needs replacing and it’s not at a time where I’ve got five minutes to do it but someone’s waiting for a test so I’ve got to run and do it … those are the things that get ‘hoha’ [bothersome, NZ Maori]. R8
Nursing staff expressed anxiety that arose from the responsibility they felt to ‘keep the machine working 24/7’ and about making mistakes with the analyser. There was a strong sense from the nursing staff of feeling they were to blame for some of the technical difficulties and setbacks:
It’s not actually that hard, and I think that’s probably a mistake we’ve made… we haven’t trained more people to be able to change those things. ... R8
The person who taught us how to use the machine made out like it was the simplest thing, and it couldn’t possibly go wrong … R15
All staff (doctors, nurses and management) were conscious of associated costs both direct and indirect and that these would determine the long-term sustainability of the haematology analyser for Hokianga.
Commitment: Despite the challenges, participants could see the benefits of the POC haematology analyser at the hospital for patient care and did not want to risk losing this. There was an overwhelming sense that everyone wanted to make it work:
I honestly think it would be good – it’s a good thing to have … we just need more training around it. We need to just be more able to fix the faults, sort it out, know what to do, so it’s not so time-consuming, and we’re just more familiar with it….. R8
We have to make this work. I am sure with the issues with the machine highlighted by the nurses can be resolved as they suggested – more training … early ordering of resources and so on . An old proverb our tupuna [ancestors, NZ Maori] have is ‘we have gone too far, to not go further, we have done too much not to do more’. R16
Part III: Cost–benefit analysis
The tangible costs of operating the haematology analyser at Hokianga Hospital were assessed at $36,500 (ex. GST) per annum. This included the depreciation on the capital cost of the analyser spread over the expected life of the asset, the consumables, staff time, training costs and overheads. An estimated 0.15 full-time equivalent of registered nurse time was allocated for the ongoing running of the analyser including the internal component of the QA program. The external component of the QA program was provided by NDHB.
The tangible benefits were difficult to calculate, but based on a conservative average cost of hospital admission (Diagnosis Related Group cost) of $2500 (ex. GST). The direct savings to the base hospital were broadly estimated to be a saving of $87,500 (ex. GST) per annum, and for Hokianga Health to be $62,500 (ex. GST) per annum. It was acknowledged that these costs for hospital services are largely ‘sunk’ costs, where a significant proportion of the hospital costs are associated with the fixed infrastructure. Savings from reduced demand occur over a longer period.
The intangible benefits of having an FBC available at Hokianga included culturally appropriate care provided closer to home; clinicians being able to provide appropriate patient care without moving patients unnecessarily, thereby reducing stress and costs on patients and families; improved communication and integration of patient care between hospitals; and improved job satisfaction for clinicians.
Discussion
This research is, to the authors’ knowledge, the first published evaluation of a POC FBC analyser in an acute clinical care setting with no onsite laboratory.
POC haematology tests increased diagnostic certainty and significantly altered patient disposition by reducing transfers to the base hospital, increasing discharges home and allowing more patients to be managed appropriately locally. The FBC result informed discussion both between clinicians and patients and between the rural generalists and the receiving urban-based specialists, facilitating a more seamless patient journey. Overall healthcare costs were reduced.
These results have added relevance from a cultural perspective. For Maori, relationships with whenua (land and identity, NZ Maori), whanau (family contribution to their health, NZ Maori) and tupuna (elders who provide security, wisdom and guidance, NZ Maori) are intrinsic to wellbeing. Maori are nurtured within this living environment of sustainability, valuing the security provided by whanau in their role as kaitiaki o te kainga (caring, responsibilities at home, NZ Maori). Being moved away from their home and entrusting their lives to distant health services can cause anxiety, and disconnection from the nurturing circle of whenua, whanau, tupuna can further impact on health outcomes17.
As expected, the majority of POC haematology tests were carried out during after-hours when the turnaround time for an FBC was previously the longest, concurring with previous studies5. For rural generalists working long hours on their own and managing acute undifferentiated cases across the primary–secondary interface the added diagnostic certainty provided by an FBC improved clinical confidence.
Findings concur with previous research that the implementation of POC testing in a rural setting without a laboratory means increased workload and responsibility for frontline staff18,19. Results indicate that not adequately addressing this with the right support and resources can create an unjust burden on nursing staff. Findings also raise the issue of potential threats to POC quality assurance when clinical staff assume responsibility that may be beyond their scope of practice. This highlights the critical importance of ensuring that there is oversight of governance and robust quality management systems for POC in sites without the support of a laboratory13,20.
Findings illustrate the concept of ‘stick-at-it-iveness’, a term previously coined as a characteristic of community resilience21. Despite the challenges, local staff persevered, seeing the benefits to their patients and community, thereby demonstrating the resilience that is familiar in rural community contexts21,22.
A limitation of this study was that participants were asked to hypothesise on disposition decisions related to a blood test result, yet disposition decisions are complex, involving more than the result of a single test and include the wishes of the patient and their family, and the resources of the whole healthcare system. The relatively short study period means that factors such as an enthusiastic response to a new intervention and behavioural change due to participants being observed may have affected the study outcomes. Several of the researchers were embedded in the reality the study was aiming to understand. This was acknowledged, the researchers remaining aware of their own subjectivity by keeping reflexive notes regarding potential effects of their participation in the study. A research team member not employed at the study site was involved in the interview analysis.
The study focused on a single rural health service with a particular geography and population. This needs to be taken into account when translating findings.
While overall healthcare costs were reduced, this study highlights the hidden costs of implementing POC systems and their associated QA programs in a remote-from-laboratory context. The funding formula of laboratory services in NZ has not kept pace with the need for diagnostic support for decision making in rural and remote settings. POC technologies are still concentrated close to and tailored for large laboratories in urban areas. Rural health services often provide continuous acute care to some of the most disadvantaged communities without access to basic laboratory tests. If funded and implemented appropriately, POC testing has the potential to significantly reduce this disadvantage.
The challenge for small integrated rural health services is maintaining a whole array of quality services for comprehensive primary acute and chronic care at a distance from most diagnostic and support services: POC laboratory testing is one small component of this. The importance of funding paradigms that better support integrated and sustainable solutions for rural communities cannot be overstated23.
Conclusions
Access to an FBC through POC testing improves the quality of acute care available to the Hokianga community, is cost effective, geographically and culturally appropriate and brings care into line with that of other regional and national acute healthcare services. In doing so, it helps address inequities in health service delivery for this rural Maori community. Health services providing acute and emergency care should have timely access to the essential laboratory tests needed to provide best patient care, regardless of geographic location. Advances in POC technology are making this goal attainable.
Acknowledgements
This research was funded by the School of Medicine, University of Auckland. The authors thank the staff at Hokianga Hospital for their contributions to the study. Thanks also to Dr Win Bennett and Professor Warwick Bagg both of the Auckland School of Medicine, for their support of the study.





