Introduction
After the successful eradication of small pox, WHO launched the Global Polio Eradication Initiative in 1988 to eradicate poliomyelitis1. Mass immunization campaigns with the oral polio vaccine (OPV) in children less than 5 years of age is one of the key strategies to achieve this goal. OPV, an effective vaccine against poliomyelitis, along with acute flaccid paralysis surveillance has helped reduce the incidence of polio to a few countries. Reported cases of polio decreased from over 350 000 in 125 countries in 1988, to 905 cases and sustained circulation of wild virus to only four endemic countries (India, Pakistan, Afghanistan, Nigeria) in 20052.
Exposure to high temperatures is detrimental to OPV and the vaccine loses it potency at a rate of 4% to 13% per day at 25°C, 11% to 21% per day at 31°C, and 26% to 34% per day at 37°C3. The cold chain is a series of storage and transport links designed to preserve vaccine potency by maintaining the appropriate temperature until it reaches its recipient. The cold chain extends from the point of manufacturing to the point of administration and is governed by guidelines for temperature and equipment requirements at each level of storage and distribution.
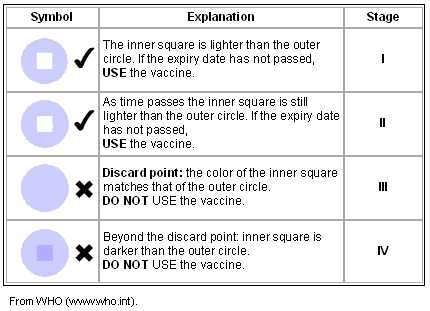
The Vaccine Vial Monitor is a small patch of heat-sensitive material placed on the vaccine vial to register cumulative heat exposure. A direct relationship exists between the rate at which the Vaccine Vial Monitor changes color and ambient temperature; the lower the temperature, the slower the color change; the higher the temperature, the more quickly the color changes4. The combined effects of time and temperature cause the Vaccine Vial Monitor to change its color gradually and irreversibly4.
As is shown (Table 1), as the Vaccine Vial Monitor changes its color from stage I to stage IV as the result of increased temperature, the user is advised against using the vaccine. The Vaccine Vial Monitor is a crucial tool to monitor potential problems with potency of OPV in the field, especially in developing countries. Poor infrastructure and insufficient resources present a number of challenges maintaining the cold chain5.
Table 1: How to read a Vaccine Vial Monitor
In WHO's South-East Asia region, seven out of 11 member states have been able to successfully interrupt transmission of wild poliovirus, with India being the only polio endemic country6. From 25 253 polio cases (types 1, 2 and 3) in 1988 to 66 laboratory confirmed cases (types 1 and 3) in 2004, India has come a long way in its battle against poliomyelitis2.
In a country with weak health infrastructure, unsanitary conditions and overcrowding, communicable diseases like poliomyelitis find a conducive environment7-9. A combination of the above factors is believed to be responsible for the increased incidence of polio cases in India from April through to June10.
A recent study evaluating the Vaccine Vial Monitor at different levels of storage in northern India attributed weaknesses in the cold chain to the loss of vaccine potency11. This study used the Vaccine Vial Monitor to evaluate the cold chain but did not include an evaluation of the cold chain equipment or temperature maintenance requirements in the research protocol11.
Jain et al. explored the relationship between health infrastructure and Vaccine Vial Monitor stage and found that most vaccine stores at district level were stage III10. On the other hand, this study failed to report any samples obtained from the sub-health centers, which are the farthest points of vaccine administration in rural India. Jain et al. also conceded their inability to comment on the status of Vaccine Vial Monitors and potency of the vaccine because potency status of the individual polio serotypes was not performed10.
Other studies have reported a knowledge deficit concerning Vaccine Vial Monitor among healthcare workers. Thakur et al. reported only 67% of health care workers being aware of the Vaccine Vial Monitor12. Another study conducted in New Delhi, India, reported 82% of the healthcare workers being familiar with the Vaccine Vial Monitor during a polio immunization drive13. Mukherjee et al. have also reported a relationship between changes in the Vaccine Vial Monitor from stage I to stage IV with failure to maintain an adequate cold chain14.
The literature provides little evidence of studies systematically evaluating the relationship between the Vaccine Vial Monitor, the cold chain equipment and the health infrastructure in rural areas of India. A review of the literature yielded two studies conducted more than a decade ago evaluating the cold-chain equipment in rural India. Aggarwal et al. reported a number of shortcomings concerning the cold chain such as power failures and improper and inadequate maintenance of cold chain equipment15. Bachani et al. reported a shortage of temperature maintenance equipment. In this study, only 58% of the sub-health centers reported having the proper carriers to transport the vaccines16.
Neither of these studies explored the relationship between the Vaccine Vial Monitor and cold chain equipment such as ice-lined refrigerators, deep freezers, and ice packs, or how the health-care infrastructure (primary, community and sub-health centers) in rural India impact on the maintenance of the Vaccine Vial Monitor.
Our study was designed to evaluate the relationship between the status of cold-chain equipment and the changes in the stage of the Vaccine Vial Monitor on the polio vaccine vials in a rural district of India.
Methods
This research did not involve human subjects and thus was exempt from individual informed consent requirements. The local health authorities that granted permission for the study requested that none of the health centers included in this study be identified.
Country-specific guidelines for cold chain maintenance (referred to as 'WHO-India protocol' from here on), developed by the Ministry of Health and Family Welfare, Government of India17, were used for evaluation of the cold chain equipment.
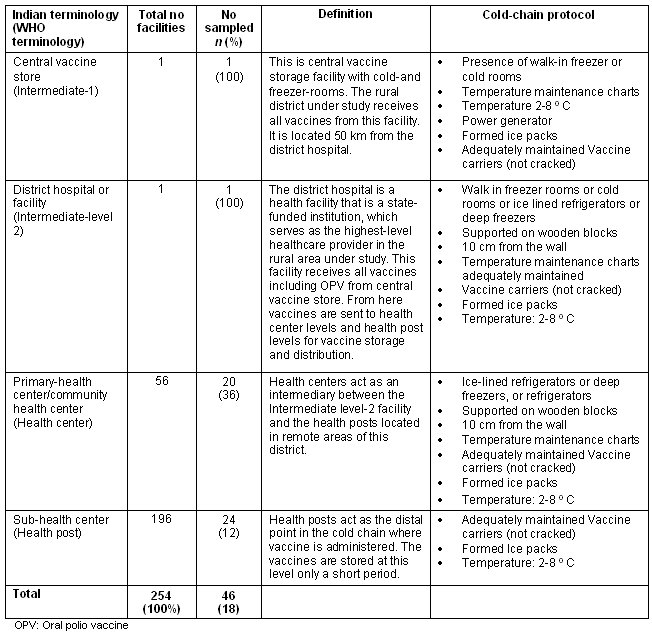
The health facilities that constitute the cold chain in rural India are defined (Table 2), along with the terminology used by the Government of India. Briefly, the central vaccine store (WHO intermediate level-1) is the storage facility with cold rooms and walk-in freezer rooms. The district hospital (WHO intermediate level-2) serves as the highest-level healthcare provider in a rural area. Primary/community health centers (WHO health center) act as an intermediary between the district hospital and the sub-health centers located in remote areas. Sub-health centers (WHO health posts) act as the farthest point in the cold chain at which the vaccine is administered. Table 2 also indicates the recommended cold-chain equipment for each level of the health center17.
Table 2: Health facilities constituting the cold chain
The WHO-India protocol recommends that each vaccine storage and distribution facility with electrically operated refrigeration equipment has a power generator to secure a reliable source of electricity, and a temperature maintenance chart17. It also recommends that ice-lined refrigerators, deep freezers and refrigerators maintain a temperature of 2° to 8° C for OPV17.
Vaccine carriers and cold boxes are non-electrical equipment used for vaccine storage and transportation. The vaccine carriers and cold boxes use ice packs to maintain an adequate temperature (ice packs must be properly frozen before they are placed in the vaccine carriers and cold boxes)17. WHO also provides guidelines for evaluation of Vaccine Vial Monitor as is shown (Table 1).
Study area and evaluation instrument
The cold chain evaluation was conducted in a rural district of central India which is served by one district hospital, 56 primary health centers/community health centers and 196 sub-health centers. Five compatible polio cases and 64 acute flaccid paralysis cases were reported in this district in 2004. All levels of health centers from central vaccine storage facility to the remote sub-health center were included for evaluation of the cold chain.
The cold chain evaluation instrument was designed using the WHO-India cold chain maintenance protocol17, and included information on demographics, cold-chain equipment, power generators and temperature monitoring charts, assessment of the set up and maintenance of electrical equipment, problems relating to frequency and seasonal trends of power failure, availability of a repair technician, and a vehicle for transporting the vaccines. For evaluation of the Vaccine Vial Monitor, another instrument was developed which included stages I to IV of the Vaccine Vial Monitor4.
Sampling
A stratified multi-stage sampling strategy was used. Phase I involved sampling of vaccine storage and distribution facilities (health centers) at each level in the cold chain (Fig 1). Phase II involved sampling equipment used to store and transport the OPV within each vaccine storage facility that was sampled in the phase I. Phase III involved sampling OPV vials from equipment which was sampled for evaluation in phase II (detailed methodology is included in Appendix I). A total of 46 health centers were evaluated for cold-chain equipment for this study.
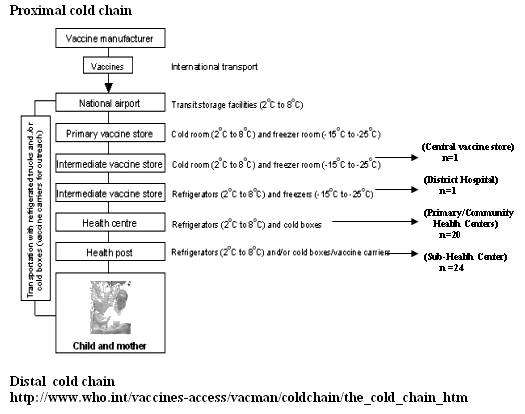
Figure 1: Pictorial representation of WHO protocol for cold chain maintenance.5
Evaluation protocol
Training in evaluation of the equipment and Vaccine Vial Monitor was provided to the evaluators by the local WHO representative and the regional district immunization officer. Data were collected over a period of 4 weeks using the cold chain evaluation instrument. Typically, only one evaluator assessed equipment at each facility. A WHO representative accompanied the evaluators to 9 health centers (20%) in order to monitor data quality and adherence to the study protocols.
The following process was followed for the evaluation of cold-chain equipment. First, the evaluators checked for the presence and maintenance of the equipment at each level in the cold chain. Second, the evaluators assessed the equipment for adequate temperature maintenance (detailed evaluation protocol in Appendix I). Third, if the facility had electrical refrigeration equipment, it was assessed for the presence and maintenance of a temperature monitoring chart and a working power generator.
Only non-electrical equipment such as vaccine carriers in use on the day of evaluation were included in the sample. Vaccine carriers were assessed for adequate maintenance (not cracked, properly fitting lids), temperature maintenance (< 8° C) and the presence of properly formed and frozen ice packs. Cold boxes were only evaluated for being present and not for temperature maintenance because they are used only in case of power failures17. Two primary/community health centers were found to be using generators because of power failure and thus we were able to obtain information on their working condition.
All vaccine vials present in the sampled cold chain equipment at a vaccine storage facility were inspected and the Vaccine Vial Monitor stage recorded. Each vaccine storage facility included in the sample was assigned a Vaccine Vial Monitor stage based on the single highest stage of Vaccine Vial Monitor found at that facility on a vial. For example, if at a primary health center A, 5 polio vaccine vials were included in the sample for evaluation of the Vaccine Vial Monitor stage, and if the highest Vaccine Vial Monitor stage observed among these 5 vials was stage II, then this primary health center A was assigned a Vaccine Vial Monitor of stage II, even if the other 4 vials read stage I.
This protocol was developed because previous studies have shown that healthcare workers in India lacked adequate knowledge to discern between various stages of the Vaccine Vial Monitor, and it is quite possible that vaccines may be administered to the children regardless of the Vaccine Vial Monitor stage12-14. Based on the findings of previous researchers, and after consultation with WHO-India field staff, we chose to assign the worst Vaccine Vial Monitor stage (as described in the preceding paragraph) found at a health center instead of the mean or median stage for the Vaccine Vial Monitor.
Data analysis
For data analysis, Vaccine Vial Monitor stage I was assigned score of 1 and Vaccine Vial Monitor stages II, III and IV were assigned a score of 0. Because there is a 34% drop in the potency of the OPV when exposed to 37° C, we decided that stage I Vaccine Vial Monitor as the only acceptable level.
For this article, variables only at primary/community health centers and sub-health centers were subjected to detailed data analysis because the data indicated that Vaccine Vial Monitor changes had taken place at these health centers. Regression analysis included only the variables that were common to both primary health center/community health center and sub-health center facilities.
Based on the WHO-India cold chain protocol for refrigeration equipment, a temperature monitoring chart, vaccine carriers and ice packs, each health center received a score of up to 100%. For example, among the 20 primary/community health centers sampled for evaluation, on average only 65% of the refrigeration equipment, temperature maintenance charts, cold boxes and power generators were present.
Vaccine Vial Monitor stage, distance from the district hospital, vaccine carrier temperature, and condition of the ice pack were the variables included in the model with Vaccine Vial Monitor stage as the outcome variable. Vaccine carrier temperature and the condition of ice packs were highly correlated (Pearson correlation coefficient = 0.556, p< 0.01).
To reduce the effect of correlation between vaccine carrier temperature and the condition of ice packs, principal component analysis was performed to transform the correlated variables into a single composite vector. Although it is not observable, this principal component vector can be interpreted as an overall measure of 'temperature' during transport. Because it captures as much of the variability of the original variables as possible, it can replace these correlated variables without much loss of information. The composite vector was used in a logistic regression model along with distance from district hospital < 30 km to find a relationship between Vaccine Vial Monitor stage and 'temperature' (condition of ice packs and temperature of vaccine carrier).
Chi-square analysis was used to examine the relationship between the type of health center and Vaccine Vial Monitor stage, and the type of health center, condition of ice packs and temperature of vaccine carriers.
Results
Forty-six vaccine storage facilities were evaluated for Vaccine Vial Monitor stages and cold-chain equipment. Of these 46 facilities, 58% (95% CI = (43.74% - 72.26%)) yielded a Vaccine Vial Monitor stage I, 33% (95% CI = (19.41% - 46.59%)) yielded Vaccine Vial Monitor stage II and 9 % (95% CI = (0.73% - 17.27%)) yielded Vaccine Vial Monitor stage III. There were no facilities with Vaccine Vial Monitor stage IV.
Central vaccine store
This facility (n = 1) was 100% compliant to the WHO-India cold chain protocol and temperature requirements. All the 25 vaccine vials sampled at this central vaccine store yielded Vaccine Vial Monitor stage I.
District hospital
This facility (n = 1) was 100% compliant in terms of electrical refrigeration equipment, temperature monitoring chart and availability of a power generator. Two of the 4 vaccine carriers sampled at this facility did not have properly formed ice packs; however, the vaccine carriers were able to maintain the required temperature. This facility yielded a Vaccine Vial Monitor stage I for the sampled vaccines.
Primary/community health centers
These facilities (n = 20) were on average 65% compliant for electrical refrigeration equipment, temperature monitoring chart, presence of cold box and availability of a power generator. Ninety-five percent of the sampled vaccine carriers had adequately maintained temperatures. Seventy-five percent of the ice packs sampled were in proper condition. Seventy-five percent of these health centers yielded Vaccine Vial Monitor stage I, 15% yielded Vaccine Vial Monitor stage II and 10% had Vaccine Vial Monitor stage III.
Sub-health centers
There were 24 sub-health centers. Only 58% of vaccine carriers maintained the required temperature and 38% of ice packs lining the sampled vaccine carriers were in proper condition. Forty-two percent of these health centers yielded Vaccine Vial Monitor stage I, 50% yielded Vaccine Vial Monitor stage II and 8% yielded Vaccine Vial Monitor stage III.
The difference in the Vaccine Vial Monitor stages I and II between primary/community health centers and sub-health centers was found to be statistically significant (p< 0.05) (Table 3.). The composite vector consisting of the vaccine carrier temperature and the condition of the ice packs was found to be highly correlated to changes in Vaccine Vial Monitor stage (from Vaccine Vial Monitor stage I to Vaccine Vial Monitor stage II) (p<0.001; OR = 6.6 (95% CI = 2.14-19.0.)).
Table 3: Relationship between level of health center and Vaccine Vial Monitor stage
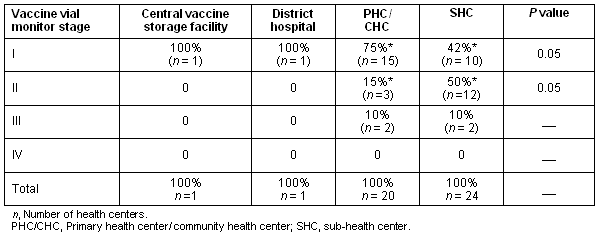
We also found that the ice packs and vaccine carriers were more likely to be in good condition and functioning at the primary/community health centers compared with the sub-health centers (p<0.01).
The relationship between distance from the district hospital of a health center and the Vaccine Vial Monitor stage was inconclusive. However, in general, we found that the farther the vaccines traveled from the district hospital, the more likely they were to change their Vaccine Vial Monitor to Stage II or Stage III (Fig 2).
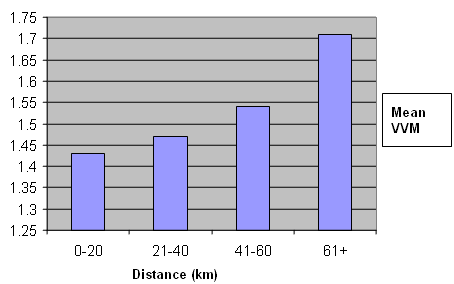
Figure 2: Relationship between the distance the vaccine traveled from the district hospital in kilometers and Vaccine Vial Monitor stage (VVM).
Discussion
Vaccine delivery problems are compounded by the rural residence of the majority of the population and weak public health infrastructure in India. It is necessary to monitor the Vaccine Vial Monitor which predicts the vaccine potency at the time of vaccine administration18.
The WHO introduced the Vaccine Vial Monitor technology for heat labile vaccines with a two-fold intent: to reduce vaccine wastage, and to identify heat-damaged stock, thus preventing less efficacious vaccines from being administered to children19.
It is critical that Vaccine Vial Monitor technology be properly utilized in rural areas of polio endemic nations where the vaccines have to travel long distances before they are administered. It is also important that the infrastructure that keeps the Vaccine Vial Monitor in proper condition be adequately maintained.
Cold chain evaluation studies in India have reported gaps in the cold chain negatively impacting the Vaccine Vial Monitor15,20,21. Our study found significant relationships between the change of Vaccine Vial Monitor from stage I to stage II or III and health infrastructure, and the change in Vaccine Vial Monitor from stage I to stage II or III and specific cold-chain equipment.
We found that ice packs and vaccine carrier temperature were the two variables that most impacted the change in Vaccine Vial Monitor stage at the primary/community health center and sub-health center levels. Poor condition and maintenance of vaccine carriers has also been reported by Goel et al., in their evaluation of cold chain for OPV in New Delhi, India22. In another study conducted by Devinayagam et al., loss of vaccine potency was significantly related to the use of improper vaccine carriers22. Similar findings concerning improper condition of ice packs and vaccine carriers were also reported by Aggarwal et al. in their evaluation of the cold chain in northern India15.
Results from our study indicate that changes in Vaccine Vial Monitors are more likely to happen at primary/community and sub-health centers than at larger facilities, such as the district hospital or central vaccine storage facility. These results suggest that remedial actions at lower or distal levels of the cold chain, addressing problems concerning maintenance of ice packs and vaccine carriers, will ensure delivery of a potent OPV.
The relationship between the distance that the vaccine vial traveled from the district hospital and change in Vaccine Vial Monitor stage was found to be statistically insignificant. However, in general, the Vaccine Vial Monitors were found to move from stage I to stage II as the distance increased (Fig 2.). This finding supports the need to strengthen the cold chain at its farthest point and improve the effectiveness of low cost measures such as the provision of adequate ice packs and vaccine carriers.
Conclusion
The Government of India and the international agencies working towards polio eradication must invest in and improve the local infrastructure in rural health centers that facilitate storage and administration of the polio vaccine.
The Vaccine Vial Monitor remains a cost-effective and pragmatic technology that ensures a potent vaccine is delivered to children. This study points to the need to specifically strengthen the health infrastructure at the distal ends of the cold chain (primary/community health center and sub-health center).
The data concerning ice packs and vaccine carriers suggest that low cost remediation of the problems identified in this study will help improve Vaccine Vial Monitor outcomes, and enable OPV and other heat-labile vaccines to maintain better potency at the time they are administered to children in remote and rural areas of India.
Acknowledgements
The authors appreciate the help and support of National Polio Surveillance Project officers in Madhya Pradesh India in accomplishing this project. We would also like to thank WHO India for its help in facilitating the data collection procedures for this project. This research effort was funded by the School of Public Health, University of Minnesota, MN, USA.
References
1. WHO. Global polio eradication initiative: eradication strategies. (Online) no date. Available: http://www.polioeradication.org/strategies.asp (Accessed 1 February 2007).
2. WHO. Wild polio virus 2000-2006. (Online (2006). Available: http://www.polioeradication.org/content/general/casecount.pdf (Accessed 1 February 2007).
3. WHO. Report of Expert Committee on Biological Standardization - investigation of the thermal stability of current oral poliovirus vaccines. Preliminary summary of results. Geneva: WHO, 1989.
4. WHO. Temperature monitors for vaccines and the cold chain. (Online) 1999. Available: http://www.who.int/vaccines-documents/DocsPDF/www9804.pdf#search='four%20stages%20of%20Vaccine%20vial%20monitor' (Accessed 1 February 2007).
5. WHO. Temperature monitors for the vaccine and the cold chain. (Online) 1999. Available: http://www.who.int/vaccines-documents/DocsPDF/www9804.pdf(Accessed 6 February 2007).
6. WHO. Immunization and vaccine development: polio eradication. Available: http://www.searo.who.int/EN/Section1226/Section1228.htm (Accessed 6 February 2007).
7. Mehra M, Bansal Y. Prevalence of paralytic poliomyelitis in a rural and urban community of Delhi. Indian Pediatrics 1990; 27: 915-917.
8. Varghese M, Qadeer I, Mohan D. Paralytic poliomyelitis in a rural area of north India. National Medical Journal of India 1997; 10: 8-10.
9. Das A. The cost of eradicating polio. (Online) 2005. Available: http://indiatogether.org/2005/apr/hlt-poliocost.htm (Accessed 1 February 2007).
10. National Pulse Polio Surveillance Project. Polio Data (2002-2005). (Online) no date. Available: http://npspindia.org/bulletin.pdf (Accessed 1 February 2007).
11. Jain R, Sahu AK, Tewari S, Malik N, Singh S, Khare S. Cold chain monitoring of OPV at transit levels in India: correlation of VVM and potency status. Biologicals 2003; 31: 237-244.
12. Thakur JS, Swami HM, Bhatia SP. Staff awareness of oral polio Vaccine Vial Monitor in Chandigarh. Indian Journal of Pediatrics 2000; 67: 253-254.
13. Lal P, Malhotra R, Gautam VP, Mehra M. Assessment of functioning of Pulse Polio Kendras and house to house activity in Delhi: is there any scope for improvement? Journal of Common Diseases 2003; 35: 266-272.
14. Mukherjee.A, Ahluwalia TP, Gaur LN, Mittal R, Kambo J, Saxena NC et al. Assessment of vaccine wastage during a pulse polio immunization programme in India. Journal of Health Population and Nutrition 2004; 22: 13-18.
15. Aggarwal A, Singh AJ. Evaluation of cold chain system in rural areas of Haryana. Indian Pediatrics 1995; 32: 31-34.
16. Bachani D, Bansal RD. Logistics management in Universal Immunisation Programme. Indian Journal of Public Health 1990; 34: 179-184.
17. WHO India. Operations Guide for Program Managers. (Online) 2002. Available: http://www.whoindia.org/LinkFiles/Hepatitis_B_Operations_Guide.pdf (Accessed 1 February 2007).
18. John TJ. Vaccine stability in the context of vaccine delivery in a developing country: India. Developmental Biology Stand 1996; 87: 19-25.
19. Global Alliance for Vaccines and Immunization Alliance. Vaccine Vial Monitors: Is the waiting almost over?. (Online) 2006. Available: http://www.gavialliance.org/Resources_Documents/Immunization_Focus/Download/vvm_072003.php (Accessed 1 February 2007).
20. Deshpande JM, Rao VK, Nadkarni SS, Bhatia JP, Rodriques JJ. An evaluation of cold chain in Maharashtra & Karnataka states by potency testing of field samples of oral poliovirus vaccine. Indian Journal of Medical Research 1995; 102: 60-65.
21. Goel NK, Swami HM, Bhatia SP. Evaluation of cold chain system in Chandigarh during PPI campaign 2001-2002. Indian Journal of Public Health 2004; 48: 200-204. 22. Devinayagam N, Vasudevan S, Ashok TP, Ahmed SS. Potency of oral polio vaccine stored at distribution centers in Madras. Indian Journal of Pediatrics 1990; 57: 757-761.
Abstract
Introduction:
The potency of oral polio vaccine (OPV), a heat-labile vaccine, is preserved by the cold chain. The Vaccine Vial Monitor, a heat-sensitive label, is critical to the monitoring and maintenance of the cold chain. This study was conducted to evaluate the relationship between the adequacy of cold chain infrastructure and the proper use of Vaccine Vial Monitor in a rural district of India.Methods: Forty-six health centers in a rural district were included in our evaluation of the cold chain equipment and the Vaccine Vial Monitors. Cold chain equipment and vaccine vials within each health center were evaluated for adherence to WHO cold chain maintenance protocols and the Vaccine Vial Monitor stage, respectively.
Results: Among the 46 health centers, Vaccine Vial Monitor stage I was found at 58% of the health centers, 33% of the health centers reported stage II and 9% reported a stage III, indicating weaknesses in the cold chain mechanism
Conclusion: Cold chain for the OPV was not adequately maintained at primary and sub-health centers in this rural district. Well maintained ice packs and vaccine carriers will help ensure delivery and availability of a safe and potent vaccine to children in rural areas of India.
Key words: cold chain infrastructure, India, oral polio vaccine, Vaccine Vial Monitors.
You might also be interested in:
2010 - Transfer of learning to the nursing clinical practice setting
2008 - Rural nurse job satisfaction

