Introduction
Tuberculosis (TB) remains a disease of public health significance, with an estimated 10 million cases diagnosed in 20191. Australia’s TB incidence is considered low at 5.5/100 000 population2; however, TB disproportionately affects Indigenous Australian populations, and the incidence rate in the Torres Strait in 2014 was 107/100 000 population (based on 2011 Census population data). In Papua New Guinea (PNG), TB is the leading cause of death from an infectious agent, with a TB case notification rate of 674/100 000 population in the Western Province in 20163. In 2014, the PNG National Department of Health declared Daru Island in the Western Province a ‘hot-spot’ – of approximately 500 cases of TB diagnosed on the island each year, one in ten TB cases were multidrug-resistant (MDR)4.
Disease control is impeded when DR-TB is poorly managed. DR-TB can be community-acquired or be the result of irregular or interrupted treatment, inadequate drug regimens or malabsorption of medication5,6. Most types of DR-TB, including rifampicin-resistant (RR) and MDR-TB, require second-line drugs to treat; these are more expensive, toxic, and the length of treatment is time-consuming7. MDR-TB is TB that is resistant to both isoniazid and rifampicin, two of the most potent TB drugs8. Two major factors of treatment success for DR-TB patients are prompt diagnosis and commencement of effective sustained treatment6. Delays in treatment commencement can result in high bacillary load with increased infectiousness and spread9. Early effective treatment reduces the bacterial burden and limits transmission8.
The Australian National TB Advisory Committee considers time to effective treatment commencement to be one of the most important markers of effective TB control programs10. In settings with excessive delays from symptom onset to treatment commencement, higher drug resistance is expected11. Currently, there is no global consensus on how to define excessive delay from self-reported symptom onset to treatment commencement. In principle it is important to minimise treatment delay to limit transmission risk12,13. Patient factors such as reduced health-seeking behaviours due to poor knowledge, distrust or other impediments may delay presentation13,14. Factors related to the health system such as poor TB awareness by staff, or a lack of appropriate laboratory facilities, as well as radiology and treatment access, can also contribute to treatment delay14,15. Individual and health system related factors that lead to delays should be monitored and addressed by TB control units10.
Geographical and geopolitical challenges in the Torres Strait–PNG region create healthcare disparities that affect diagnosis, referral pathways, treatment and management of TB patients in the Torres Strait Protected Zone (TSPZ). Since the ratification of the Torres Strait Treaty in 1985, free bidirectional movement has been allowed for traditional, family, economic and trade purposes in the TSPZ between 13 PNG Treaty villages in the Western Province and 13 adjacent Australian Torres Strait Islands16,17. As a result of this international agreement, TB has often been able to move, unhindered, across borders18. Primary transmission of TB and the rise of drug resistance are cause for concern for at-risk Australian and PNG border communities19. The Torres Strait Treaty does not include access to Australian healthcare for PNG villagers living adjacent to the Australian border, despite the narrow (4.7 km) stretch of water separating the two countries (Fig1). In response to the urgent nature of some healthcare needs, Queensland Health provides humanitarian aid to those most in need as well as providing triaging and point-of-care diagnostic services.
Optimising models of care for TB patients living in the Torres Strait–PNG international border region has been challenging, and the programmatic TB response in this remote area has evolved over time (Fig2). Although Torres Strait and PNG cross-border communities are subject to a similar TB risk they do not have access to the same level of health care20. On the Australian side of the border, high-risk screening and passive TB case finding is standard practice whereby cases are diagnosed post-investigation of TB-related symptoms or incidentally while other medical conditions are being investigated10,21. During the study period, PNG nationals requiring TB work-up who did not present to an Australian health facility needed to travel to Daru General Hospital, located at least 2 hours away by small motorised boat (at a cost of A$240)22. The region’s geography and associated travel costs renders accessing TB health are services prohibitive for PNG nationals residing adjacent to the TSPZ. The Multi-dimensional Poverty Index in the area is estimated to be 0.3223. Over the past 20 years, poverty has affected time to treatment commencement for PNG residents with TB living adjacent to the Australian border. The consequences are sustained transmission risk and rapid growth of DR-TB on both sides of the border.
Time from self-reported onset of symptoms to effective treatment commencement has not previously been investigated in this particular context. This study aimed to explore total time from self-reported onset of TB symptoms to effective treatment commencement for all DR-TB cases diagnosed in the Torres Strait–PNG border region over two decades. This study identifies factors associated with the time between self-reported symptom onset to effective treatment commencement as well as discusses programmatic interventions that may have contributed to reduced treatment delays over time. As DR-TB transmission is an established risk in the Torres Strait–PNG border, this study may help to improve programmatic management of TB in the region to the benefit of both Australian and PNG healthcare systems and local communities.
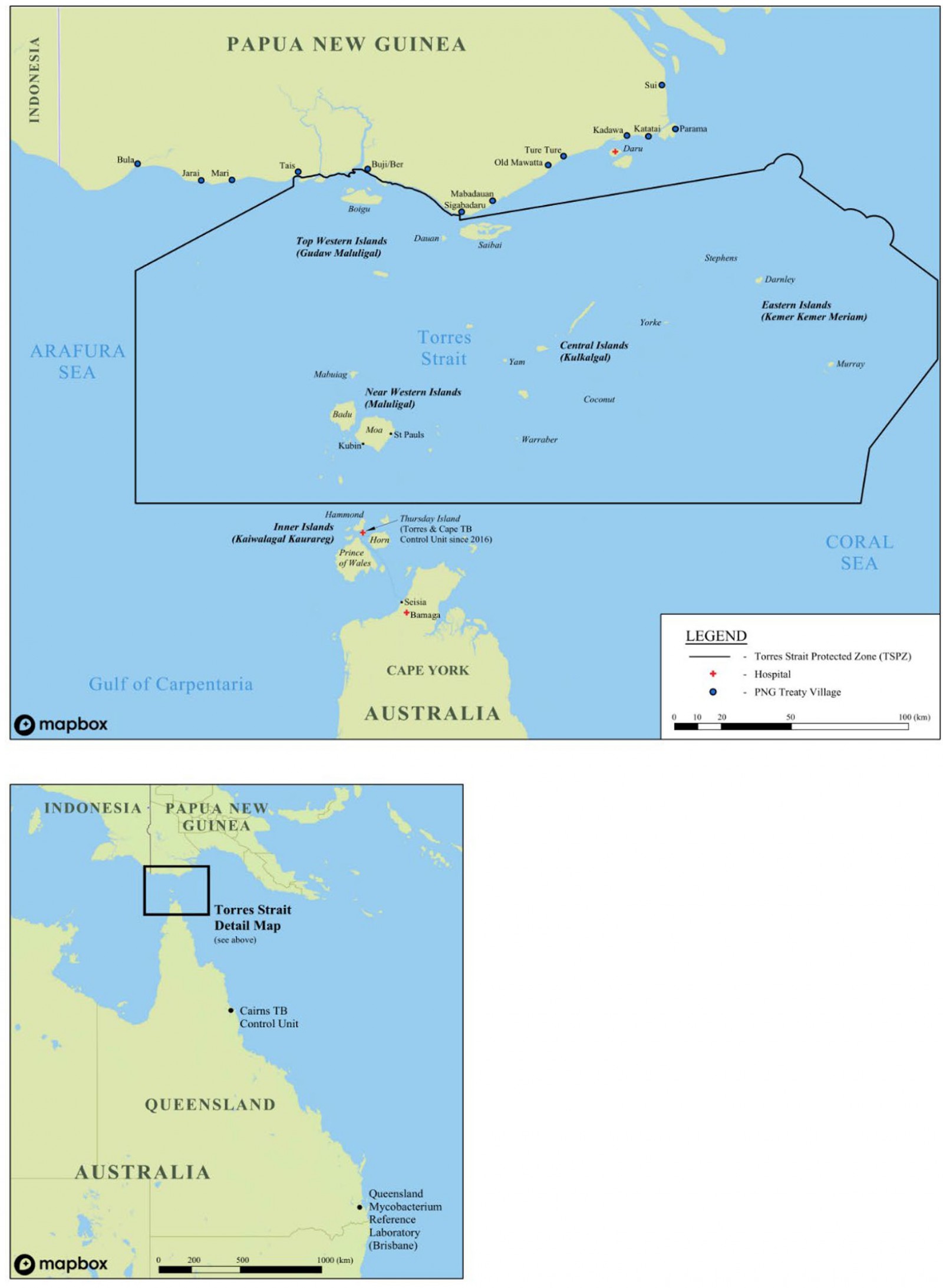 Figure 1: Map of Torres Strait–Papua New Guinea cross-border region.
Figure 1: Map of Torres Strait–Papua New Guinea cross-border region.
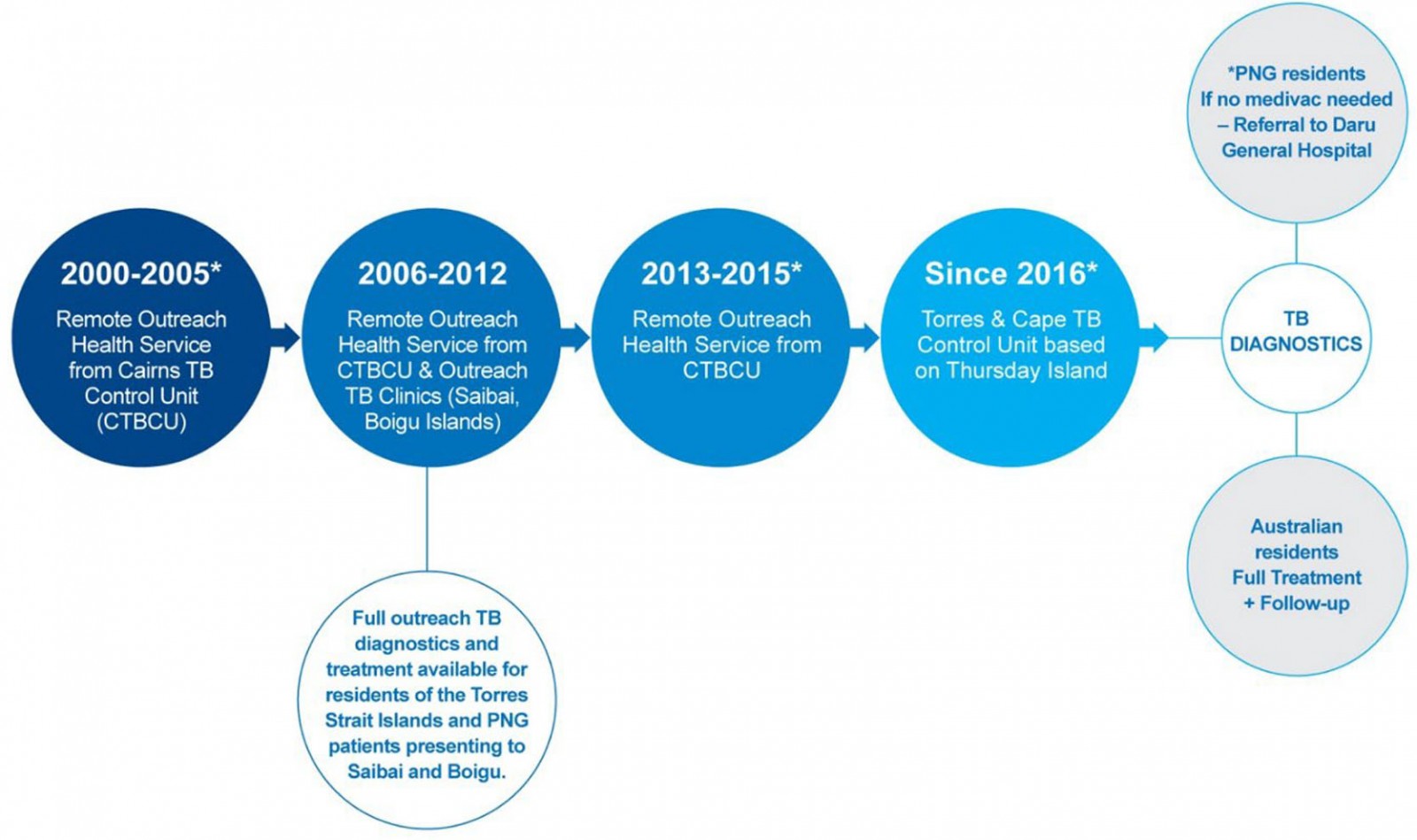 Figure 2: Description of different tuberculosis programmatic time periods in Torres Strait–Papua New Guinea cross-border region, 2000–2020.
Figure 2: Description of different tuberculosis programmatic time periods in Torres Strait–Papua New Guinea cross-border region, 2000–2020.
Methods
Design and study population
This retrospective cohort study included all patients from Australia or PNG who were diagnosed in the Torres Strait Islands with microbiologically confirmed DR-TB between 1 March 2000 and 31 March 2020. Patients of all ages, including children, were included in the study, as were those with pulmonary, extrapulmonary, smear-negative and smear-positive disease.
Sputum and other histological samples were collected in the Torres Strait Islands and transported via sea and air to a WHO-designated Supranational Reference Laboratory, the Queensland Mycobacterium Reference Laboratory (QMRL)24 (Fig1). Confirmation of DR-TB diagnosis was confirmed by culture and phenotypic drug susceptibility testing (DST) pre-November 2010, or by Cepheid Xpert® MTB/Rif Assay (Xpert) (rapid diagnostic technology), culture and DST post-November 201024.
Definitions
The sample size is based on 133 cases with DR-TB, with 113 experiencing the event (known effective treatment commencement).
Effective treatment was defined as the implementation of appropriate second-line TB treatment for RR/MDR-TB cases and appropriate TB treatment for other drug-resistant cases, whether this was programmatic/empiric or personalised.
Time to event was defined as the sum in days of the following three distinct intervals:
- patient delay: time from self-reported TB symptom onset to presentation at a health facility in the Torres Strait
- health system delay: time from presentation at a health facility to being diagnosed with TB
- treatment delay: time from diagnosis to event – effective treatment commencement.
As not all participants commenced treatment, the data are right-censored. The 20 censored cases that were transferred out or died were observed in the study to pass through the sum (in days) of patient delay and health system delay – time from self-reported onset of symptoms to diagnosis.
Diagnosis year group reflects the changes in programmatic TB responses in the remote Torres Strait–PNG international border that occurred over time (Fig2).
Case type was defined as either a new case, or relapsed case following full or partial treatment in Australia or overseas. These cases were analysed as independent events. Four patients who had previously received full or partial treatment were diagnosed twice in this study, and were counted discretely as separate events. Self-reported onset of symptoms did not overlap for any of these four patients.
Data collection
Clinical and demographic data were sourced from the Queensland Notifiable Conditions System (NoCS), and drug resistance for each case was verified in Queensland Health’s laboratory results software, AUSLAB. Case notifications were cross-checked between NoCS and AUSLAB, and one case of DR-TB identified from NoCS, but not from AUSLAB, was removed from the study.
The date of symptom onset and treatment commencement in NoCS was cross-checked against self-reported symptom onset date and treatment commencement documented in patient charts, Queensland Health’s statewide patient database, The Viewer25, and in the Torres and Cape Hospital and Health Service (North) patient database, Best Practice26. As NoCS relied on retrospective submissions of case notification data throughout the study period, original or copies of handwritten progress notes in patient charts, and referrals and progress reports in The Viewer and Best Practice, were considered the sources of truth for this study for symptom onset and treatment commencement. If this evidence was not available, the date of symptom onset and treatment commencement in NoCS was used.
For data pertaining to 2016 and onwards, a combination of NoCS, The Viewer, Best Practice and two Excel spreadsheets specifically used to manage patient data for residents of the Torres Strait Islands, and PNG patients diagnosed with TB, were used to ascertain self-reported date of onset of symptoms and date of treatment commencement for each case. Prior to the border closures associated with the COVID-19 pandemic, clinicians from the Torres and Cape TB Control Unit met with clinicians from the Daru TB Programme to discuss treatment commencement dates and treatment outcomes of shared cross-border patients. Further, a core component of the Torres and Cape TB Control Unit’s cross-border TB portfolio was to share new laboratory results, chest X-ray reports and household contact information with the Daru TB Programme.
Patients diagnosed with DR-TB at an Australian health facility prior to the end of 2012 commenced treatment for DR-TB in Australia because second-line drugs used to treat DR-TB were not yet available on Daru Island. Once drug-susceptibility testing and effective treatment of DR-TB were available on Daru Island, the numbers of DR-TB cases diagnosed by Australian health facilities decreased. Most patients from PNG who were diagnosed at Australian health facilities with DR-TB between 2013 and 2020 commenced treatment in PNG; however, the most critically unwell, who were transferred to either Thursday Island, Cairns or Townsville Hospitals may have commenced treatment in Australia. Data pertaining to the site at which each patient commenced effective treatment were not collected.
Data analyses
All statistical analyses were performed using SPSS v27 (IBM; http://www.spss.com).
Frequencies and percentages were calculated for categorical variables including treatment event (effective treatment commencement or transfer out/death), visa status, case type, RR-TB, MDR-TB and gender.
Tests for normality were performed for mean, median, skewness and kurtosis, and visual inspections of histograms were reviewed. Assumptions for normality were violated and data were not normally distributed. Medians and interquartile ranges (IQR) were used for data that were not normally distributed to demonstrate differences in the total time to effective treatment commencement across the four different time periods. When determining median days to effective treatment commencement, patients who did not commence known treatment, and were transferred out or died, were removed from analyses – hence time to treatment outcomes should be interpreted as conditional on treatment commencement.
To determine any association between advanced diagnostic technology implementation in Queensland from November 2010, a non-parametric independent samples Median Test for k samples was used. The dependent variable was total time in days to known treatment commencement and the independent categorical variables were pre and post-Xpert. Patients who did not commence effective TB treatment due to death or transfer out were removed from the analyses.
Independent samples median test of frequencies was used to identify the grand median, and to demonstrate frequencies above and below the median days to effective treatment commencement across different time periods. Post-hoc analyses were performed using pairwise comparison to determine the differences of median delays across the four different time periods, reflecting different programmatic management types. The dependent variable was total time to event (symptom onset to effective treatment commencement), and two time periods were compared at a time using pairwise comparison. Statistical significance was set at p<0.05. Due to the relatively small sample size and to counter a potential type 1 error that may occur when multiple analyses are performed on the same dependent variable, a Bonferri correction was applied27. The alpha was lowered by taking the unadjusted p-value and multiplying it by the number of pairwise comparisons27.
A Cox proportional hazards regression plot was used to illustrate the time to known treatment commencement in days, stratified by case type (new versus previous full or partial treatment).
Ethics approval
This study was conducted with ethics approval from the Far North Queensland Human Research Ethics Committee (HREC) (HREC/17/QCH/74-1157), the Chair of James Cook University HREC (H7380) and a Public Health Act approval (QCH/36155 – 1157).
Results
Descriptive statistics
Table 1 shows that of 133 patients diagnosed with DR-TB between 2000 and 2020, 20% had previously received full or partial TB treatment in Australia or overseas and 70% were RR. The proportion of patients with MDR-TB overall who previously received full or partial treatment was 26% (23/89 cases). Between 2000 and 2005, 100% of DR-TB cases were new and, of those, 71% were RR.
Table 2 shows that the median time to effective treatment commencement for 113 patients increased after 2000–2005 (92 days; IQR 70–201) and peaked between 2006 and 2012 at 138 days (IQR 72–251). Reduction to 29 days (IQR 7–45) was observed between 2016 and 2020.
Table 1: Characteristics of drug-resistant tuberculosis patients diagnosed during different programmatic time period in Torres Strait–Papua New Guinea cross-border region, 2000–2020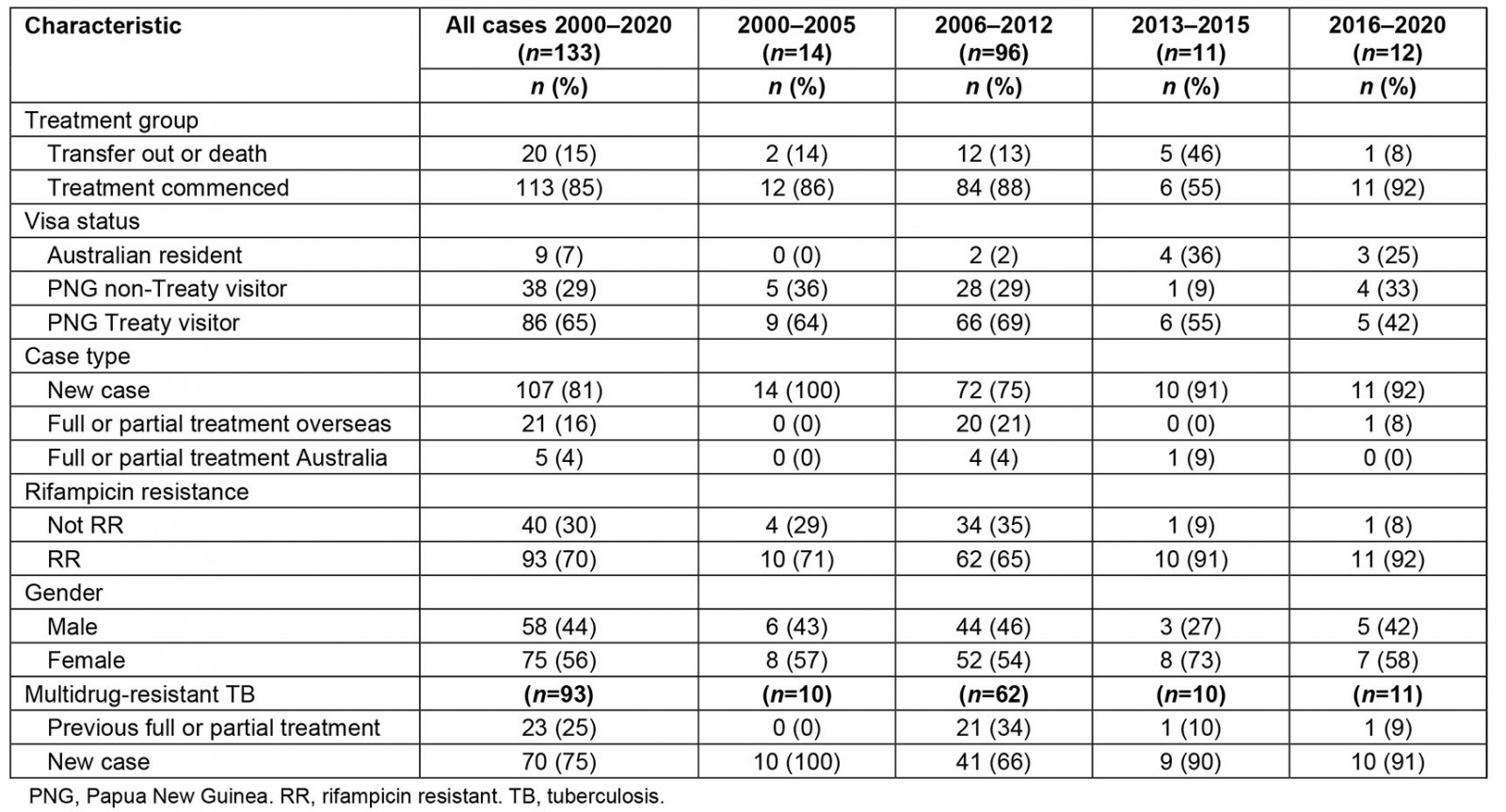
Table 2: Time to commencement of effective drug-resistant tuberculosis treatment across different tuberculosis programmatic time periods in Torres Strait–Papua New Guinea cross-border region, 2000–2020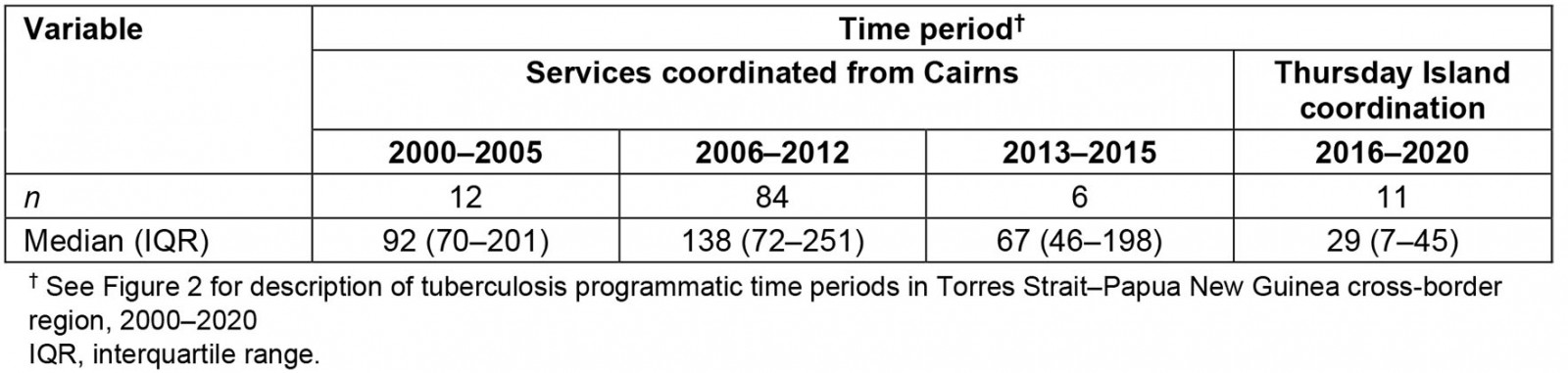
Inferential statistics
The independent samples median test (Fig3) shows that the grand median time from self-reported TB symptom onset to known effective treatment commencement for DR-TB cases was 124 days (IQR 51–214). Figure 3 shows post-hoc pairwise analyses demonstrating a statistically significant difference between the ‘time to known effective treatment commencement’ medians of patients in the diagnosis year groups 2000–2005 and 2016–2020 (p=0.04), and year groups 2006–2012 and 2016–2020 (p<0.001). Frequencies of cases both above and below the median are illustrated in Figure 3. Fifty-seven percent (n=48) of cases diagnosed between 2006 and 2012 exceeded the median total time to effective treatment commencement. All other diagnosis year groups had greater numbers of participants below the median than above the median.
The independent samples median test (Table 3) demonstrates that post-implementation of the rapid diagnostic technology, Xpert, the median time from self-reported symptom onset to treatment commencement was 67 days (IQR 30–202) and the pre-Xpert time to treat median was 135 days (IQR 73–248; p=0.07).
Figure 4a shows the Cox proportional hazard plot for case type and total time to commencement of effective treatment. As can be seen in this figure, patients who had previously received full or partial treatment demonstrated a more gradual slope to hazard (ie ‘time to known commencement of effective treatment’). Case type emerged as a predictor of earlier effective treatment commencement (hazard ratio = 0.6 (95% confidence interval (CI) 0.35–0.89); p=0.01). Figure 4b shows the Cox proportional hazard plot for TB services provided by Cairns TB Control Unit and the Torres and Cape TB Control Unit. This figure shows that patients treated within the Cairns TB Control Unit demonstrated a more gradual slope to hazard (ie ‘time to known commencement of effective treatment’).
Table 3: Time to commencement of effective drug-resistant tuberculosis treatment pre- and post-Xpert availability in Torres Strait-Papua New Guinea cross-border region, 2000-2020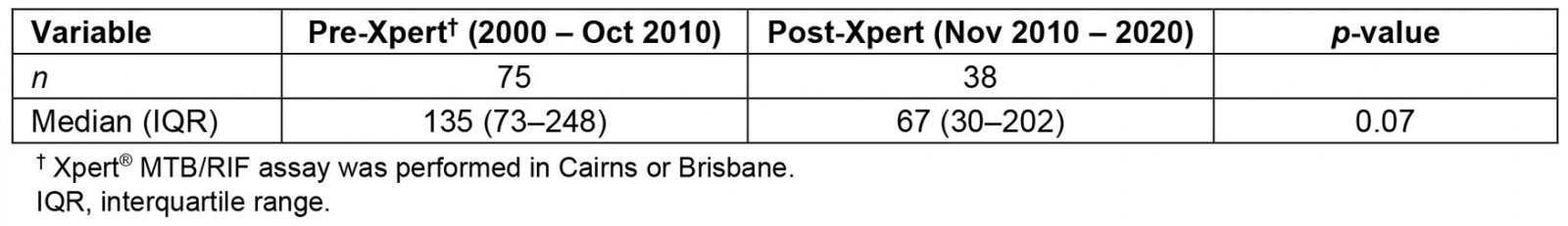
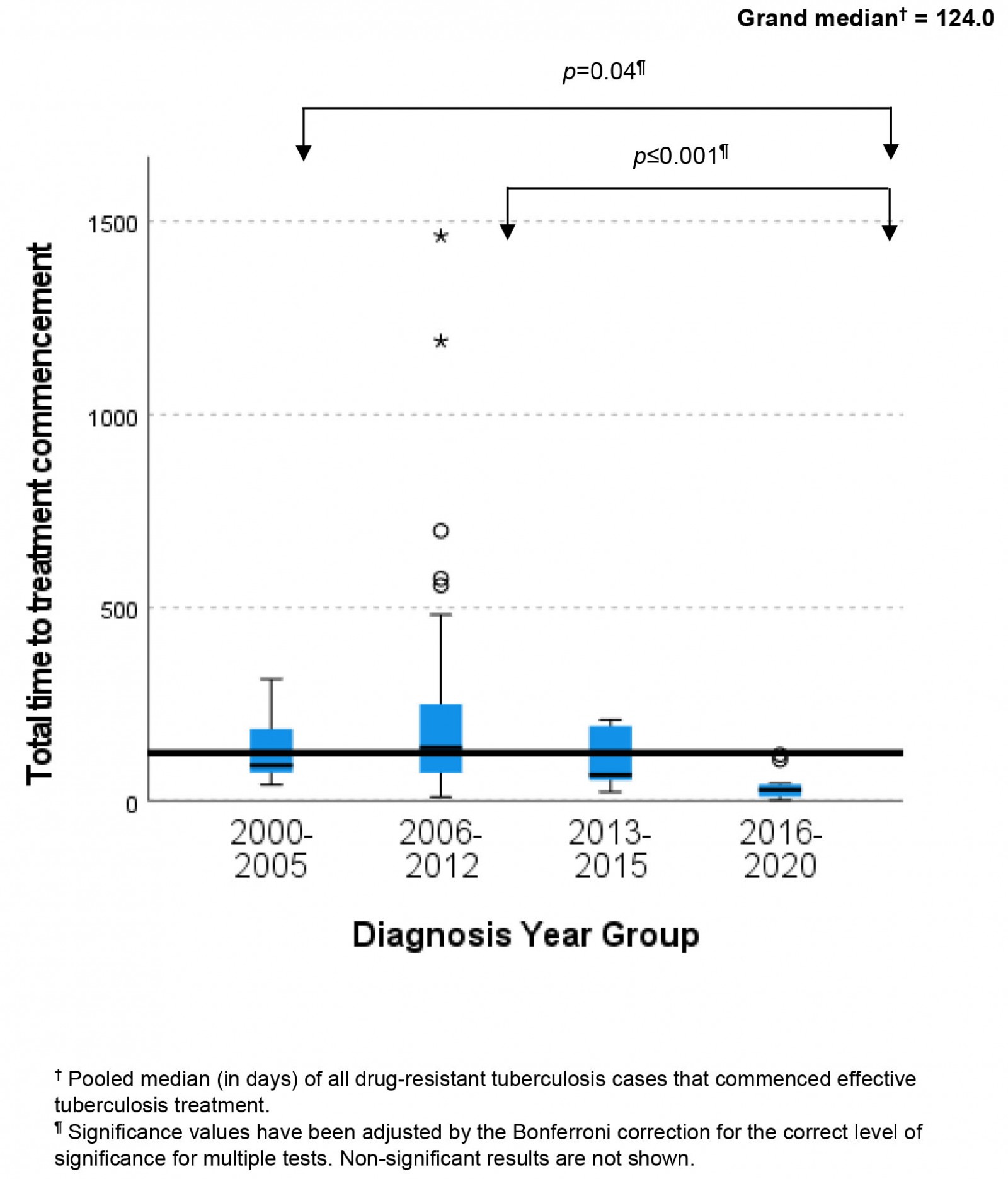 Figure 3: Variability in median time to treatment commencement across different tuberculosis programmatic time periods in Torres Strait–Papua New Guinea cross-border region, 2000–2020.
Figure 3: Variability in median time to treatment commencement across different tuberculosis programmatic time periods in Torres Strait–Papua New Guinea cross-border region, 2000–2020.
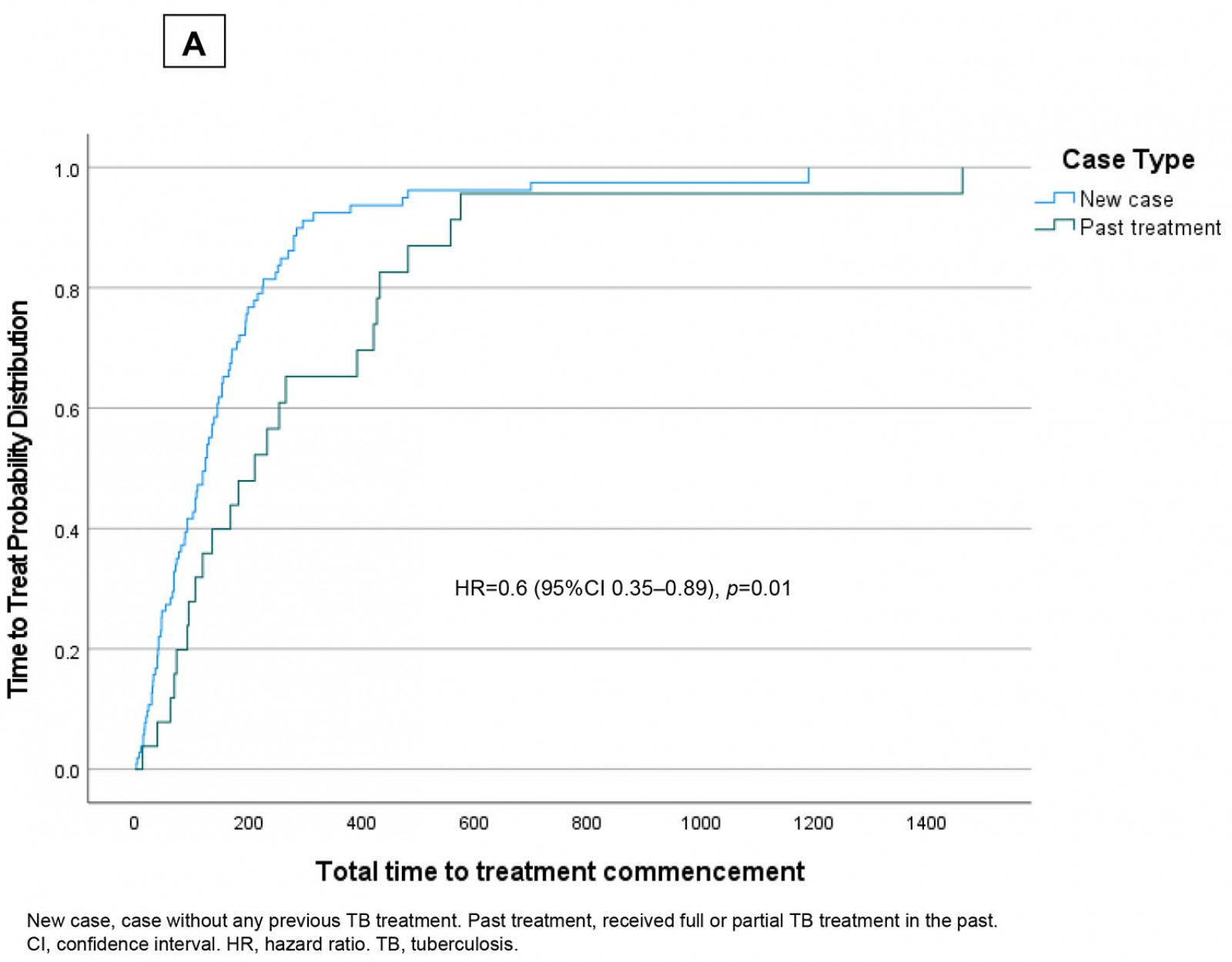
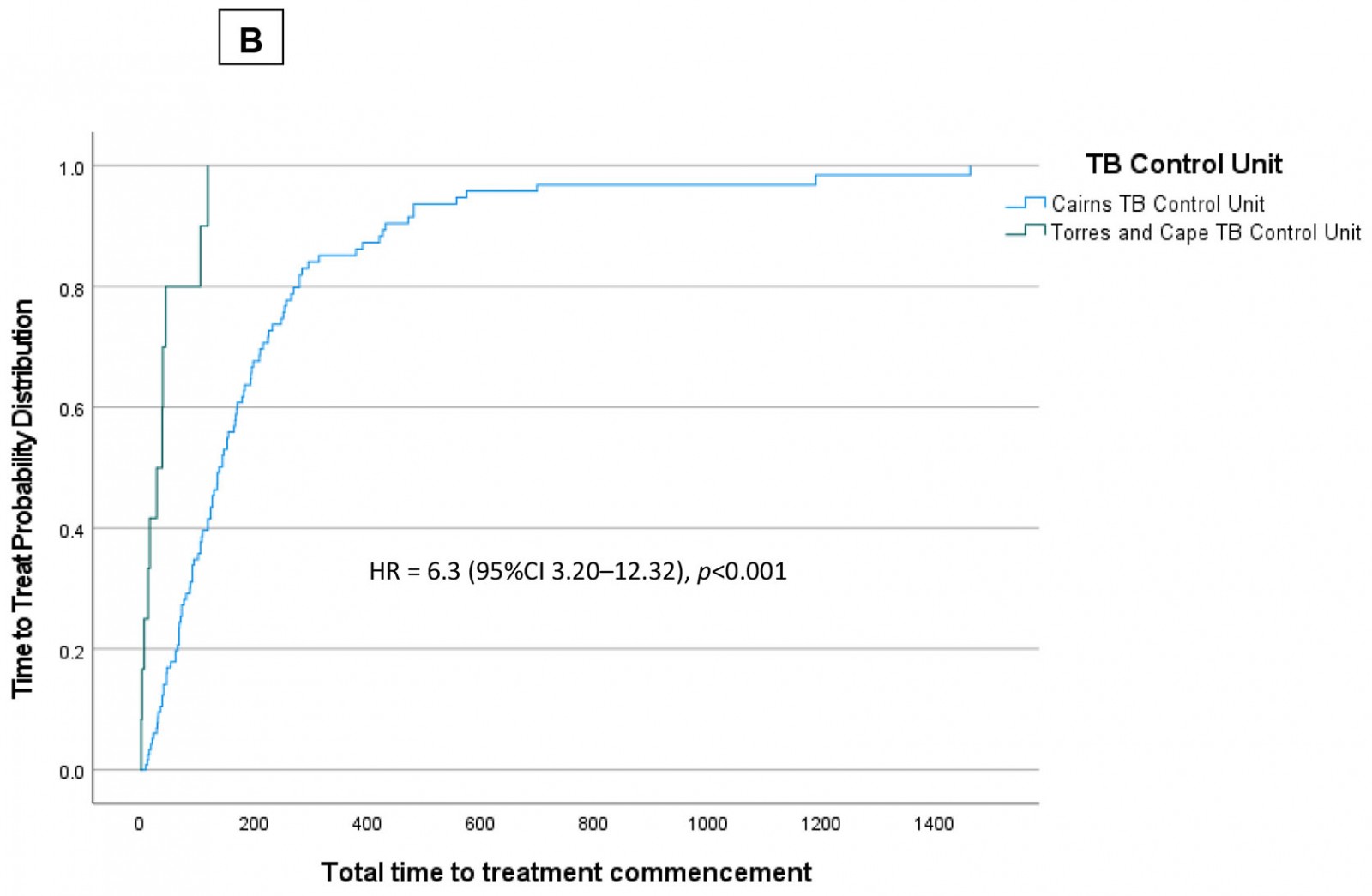 Figure 4: Time to treat (days) in Torres Strait–Papua New Guinea cross-border region, 2000–2020, stratified by (a) ‘new case’ and ‘past treatment’; (b) tuberculosis services provided by the Cairns Tuberculosis Control Unit and Torres and Cape Tuberculosis Control Unit.
Figure 4: Time to treat (days) in Torres Strait–Papua New Guinea cross-border region, 2000–2020, stratified by (a) ‘new case’ and ‘past treatment’; (b) tuberculosis services provided by the Cairns Tuberculosis Control Unit and Torres and Cape Tuberculosis Control Unit.
Discussion
In this study, the lengthy median delay from self-reported onset of symptoms to effective treatment commencement was 124 days (IQR 51–214) over the study period. This is approximately four times more than the time to treat in a study conducted in the late 1990s in metropolitan Victoria, Australia, where 33 days from symptom onset to treatment commencement was deemed an acceptable delay28. Even considering remoteness, this key finding is still well above delays in other similar settings, and the present study authors offer 4–6 weeks as an acceptable delay, based on results from Australian literature. A systematic review of 198 studies reported a pooled mean total delay of 88 days across low- and middle-income countries in both urban and remote settings12. Remoteness of the region, diagnostic services located on mainland Australia, access to pharmaceuticals for patients who commenced treatment in Australia and limited telecommunications with residents of Treaty and non-Treaty villages in PNG all remained unchanged throughout the study period, therefore variation of time to effective treatment is likely to be associated with other factors. These may be low level of health service coverage and lack of awareness of TB, as previously identified in other rural and remote settings14.
Excessive delays between onset of symptoms and effective treatment commencement may contribute to ongoing transmission of TB in the region15, hence various programmatic initiatives were introduced by the Torres and Cape TB Control Unit to strive to reduce this time. For example, culturally competent health promotion has been shown to increase knowledge and uptake of disease-prevention strategies in culturally diverse communities29. Initiatives introduced by the Torres and Cape TB Control Unit included specific onboarding and opportunistic education for staff, and development and distribution of linguistically and culturally appropriate TB education materials. These initiatives may have been contributing factors to the decrease in time to effective treatment (29 days) from 2016 to below the level considered acceptable in metropolitan Victoria (33 days)28. These contributing factors may require further investigation to better understand their effect on time to treatment.
Changes to the management of TB in the region over time may help to explain a near five-fold decrease in the median time to treat between 2006 to 2012 and 2016 to 2020. By 2012, the Daru TB Program in PNG, supported by the Australian Government, had developed capacity to manage both fully susceptible and DR-TB cases22. Measures introduced in the region from 2014 and programmatic changes from 2016 may have also contributed to a reduction in median time from onset of symptoms to effective treatment commencement. In 2014-2016, Australian Government funding supported the delivery of TB-clinician-led initiatives to enhance TB knowledge among local communities and healthcare facilities on both sides of the international border20,30-32. This enhanced knowledge of the basic features of TB may have assisted in reducing the time from self-reported onset of symptoms to presentation at a health facility.
During this time, Australian Government funding also supported a data integrity project33 to gather all historic PNG patient charts located on Australian Torres Strait Islands and patient data contained in an electronic medical record used prior to 2011: Holt’s database26. Data migration into best-practice software enabled clinicians to access patient information in a single electronic database. As a result of the cross-border data integrity project, from 2015 all PNG nationals presenting with signs and symptoms of TB to a primary health centre (PHC) in the TSPZ have an electronic medical record. This initiative led to increased real-time visibility of all patients in the TB care pathway, provided access to information about past TB diagnoses and past contact history dating back to the 1950s, and allowed for identification and follow-up of previously treated cases.
Notably, the absence of advanced diagnostic technology such as Cepheid Xpert® MTB/Rif Assay, which can identify RR in less than 2 hours, can add considerable time to health system related delays34. Implementation of Xpert in Queensland, Australia in November 2010 improved the overall median delay to 67 days in this study, which was approximately half the median pre-Xpert, indicating that implementation of Xpert in the Torres Strait may have helped to further reduce time to effective treatment delays35. Although the point-estimate difference pre- and post-Xpert was large, there were additional temporal trends at the time and the difference pre- and post-Xpert was not statistically significant in this study.
Outreach TB specialist services provided by staff based in Cairns prior to 2016 may have influenced time to treatment commencement. Logistical issues and inclement weather at times affected the ability for Cairns-based TB clinicians to access the Torres Strait to review patients and commence them on treatment22. In contrast, the Torres and Cape TB Control Unit established in 2016 hosts an ongoing presence of TB clinicians based in the Torres Strait and allowed for a continual service where suspected case management and treatment commencement could occur at any time.
In international border settings, strength is found in collaboration between international partners and where well-defined data sharing and referral agreements are in place36. In collaboration with the Daru TB Program, the Torres and Cape TB Control Unit implemented new procedures to enhance recognition and streamline management of symptomatic patients37. As a result of improved cross-border communication, programmatic rules and strategies were implemented from 2016 to strive for safe and ethical management of shared and referred patients. These included ensuring Daru’s readiness to receive patients discharged from Australian hospitals, timely notification of microbiological and radiological results, an agreement regarding case notifications to avoid over-reporting case numbers, and sharing patient outcome data. The strength of this cross-border collaboration is evidenced in this study by known treatment commencement dates of 11 of 12 cases from 2016.
Traditional risk factors for delays from self-reported onset of symptoms to effective treatment commencement in other settings have included limited access to health facilities, rural or remote location, and distance to diagnostics12,14. However, in this study, these factors apply to both new and previously treated cases. With few treatment supporters to administer directly observed therapy in most of the PNG Treaty villages38 and challenging cross-border communication pathways, previous compliance history and assurances of treatment compliance to follow were not always known. It should be emphasised that none of the variables in this study were highly predictive, which shows that there is huge individual patient variation in time to effective treatment commencement and no easily identifiable risk group. It is possible that treatment delays were more prominent in patients who had previously received some treatment due to a lack of information, patient counselling and education from past TB care providers39. As previously treated patients may have had negative past treatment experiences, thus delaying retreatment commencement, additional support for these patients is warranted40.
To help minimise the economic burden and improve timely access for PNG patients receiving TB care in the Torres Strait between 2006 and 2012, the Cairns TB Control Unit initiated free transport for PNG nationals accessing outreach specialist clinics on Saibai and Boigu Islands (Fig1); however, large numbers of patients failed to attend appointments22. A previous study identified that of 73 PNG MDR-TB patients diagnosed in the Torres Strait, 15 were lost to follow-up, and a further 25 had an unknown treatment outcome22. A recent study conducted on Daru Island reported longer delays to treatment commencement in symptomatic DR-TB household contacts presenting for TB screening when compared to symptomatic drug-susceptible household contacts41. This suggests that there may be country-specific risk factors that require focused support for DR-TB patients and their close contacts. It may also indicate that previously treated patients may have reduced access, a reluctance to present or have different ideologies, prompting a need for additional support for this cohort of patients.
Potential reasons for delays in previously treated cases could be that TB patients may not have been aware that they could be diagnosed twice, may have been too sick to present earlier, or may not have been experiencing any signs and symptoms at all. It is also possible that pain and adverse reactions from injectable drugs used in previous management of DR-TB acted as a deterrent for patients or their close contacts presenting earlier42, although it should be noted that only 45.8% of MDR-TB cases diagnosed in PNG nationals in this region between 2000 and 2014 received injectables22. Nevertheless, as 80% of cases were new in this study, this suggests that there may be evidence of acquired drug resistance in this population as a result of interrupted, irregular or inadequate treatment. Future qualitative research may assist TB programs in the Torres Strait to identify reasons why previously treated cases presented late in this region, and lead to improved and targeted strategies to improve early entry into the TB care pathway.
Limitations
It is possible that extensive delays in treatment commencement were attributed to repeated patient visits to PHCs, serviced by outreach rural generalist practitioners. It is possible that some symptomatic patients did not immediately enter into the TB diagnostic pathway as PHC clinicians and rural generalist practitioners may not have recognised TB as a leading differential diagnosis. As a satellite TB service was provided up until 2016, it is possible that TB clinicians were not notified of all symptomatic patients and rural generalist practitioners prescribed a trial of broad-spectrum antibiotics to first rule out lower respiratory tract infections43, as has been reported in other studies40,44. This study did not explore this possibility.
This study only captured PNG patients diagnosed by health services on the Australian side of the border as it focused on the management of DR-TB by the Australian health system only. A similar study could be conducted from the PNG health system’s perspective to capture PNG nationals diagnosed and treated by PNG health services to gain greater understanding of the overall management of DR-TB in this cross-border region. Should a study take place, a greater collaboration and linkage of data between health systems from both sides of the border would enhance the understanding of DR-TB management in the region and help improve programmatic strategies across the border.
Conclusion
Minimising diagnostic and TB treatment delay is a global priority. Managing TB in rural and remote settings requires TB programs to strive for minimal diagnostic delay with early initiation of effective treatment. The introduction of a decentralised TB management and control structure in the Torres Strait contributed to improved service delivery and a reduction in time to effective TB treatment commencement. The study’s findings suggest that time to effective treatment commencement can be reduced by improved access to advanced diagnostic technology and implementation of locally based, patient-centred initiatives. Evaluating time to effective treatment as a measure of programmatic efficacy is an important and valuable public health and quality improvement approach.

