Introduction
The rising of novel coronavirus SARS-CoV-2 in the Chinese province of Hubei in December 2019 led to the COVID-19 outbreak, and WHO declared the COVID-19 pandemic on 11 March 20201,2. By 3 March 2023, more than 676 million cases and 6.8 million deaths had been reported, with the Americas one of the most affected regions, with millions of cases and deaths. For instance, Brazil and the US alone accounted for more than 1.8 million deaths3.
In Ecuador, more than 1 million of cases and 36 000 deaths were reported up to March 20233. The first COVID-19 case reported in the country was identified in Guayaquil on 29 February 2020, with an elderly woman who arrived on a flight from Spain. During the following weeks, the transmission of SARS-CoV-2 across the country grew exponentially, overwhelming the Ecuadorian healthcare system. The most dramatic consequences were observed in Guayaquil during April 2020, when images of corpses in the streets of low-income neighborhoods were broadcast worldwide. All of this happened despite the efforts of the Ecuadorian government to contain the SARS-CoV-2 virus spread with a complete lockdown for all but essential services such as pharmacies, groceries, security and health services, and mobility restrictions4. Although those measures helped to control COVID-19 transmission, once the measures were relaxed in July 2020, to avoid the collapse of the economy, new COVID-19 outbreaks were observed countrywide, leading to uncontrolled community transmission5-9.
However, the epidemiological scenario was different for one of the provinces of Ecuador, the Galapagos Islands. Although the first case of COVID-19 was reported as early as March 2020, a low prevalence of and deaths associated with COVID-19 were maintained during the first wave of the pandemic. The Galapagos Islands are 972 km from the Ecuadorian coast and comprise 13 main islands, of which only four are inhabited. The Galapagos Islands have 25 124 inhabitants, distributed on Santa Cruz (population 15 393), San Cristobal (population 7330), Isabela (population 2256) and Floreana (population 145)10. The political organization of the population is in three cantons with population densities (individuals/km2) of 8.8, 8.5 and 0.4 for Santa Cruz, San Cristobal (this canton includes Floreana as a rural parish) and Isabela, respectively. Because 98% of the Galapagos Island area is national park, human population is concentrated in a main town in each of the four populated islands, and a few rural parishes. The demographic characteristics in Galapagos are very similar to continental Ecuador, with a mean age of 29 years according to the most recent national census11. Galapagos Islands health infrastructure comprises primary health facilities such as health centers, a basic hospital in Santa Cruz Island and a general hospital in San Cristobal Islands; overall, the public health system in the Galapagos has the issues usually associated with low- and middle-income countries, and the situation is aggravated by the geographic location remote from mainland Ecuador, where more specialized hospital facilities are located.
By 6 April 2020 there was a SARS-CoV-2 testing laboratory on site in the Galapagos, due to the inter-institutional collaboration between the regional government of Galapagos Islands (Consejo de Gobierno), the local authorities (Gobiernos Autonomos Descentralizados de Santa Cruz, San Cristobal and Isabela), the regional authorities from the Ecuadorian Ministry of Health, the Agencia de Regulación y Control para la Bioseguridad y Cuarentena para Galápagos and Universidad de Las Américas. By contrast, in continental Ecuador the only laboratories within the public health system in the early stages of the COVID-19 outbreak were located at the facilities of the Instituto Nacional de Salud Publica e Investigación Leopoldo Izquieta Pérez in the three main cities of Ecuador (Quito, Guayaquil and Cuenca) to attend a population of 17 million of Ecuadorians.
The present study is a retrospective analysis of the SARS-CoV-2 surveillance testing carried out in the Galapagos Islands to describe and evaluate the epidemiological situation of the COVID-19 pandemic in this remote and isolated iconic location, to prove the utility of massive testing of community dwelling individuals to control the spread of SARS-CoV-2.
Methods
Study design and setting
Two separate screenings were performed during April–May 2020 and September 2020 in the Galapagos Islands. The first sampling included only molecular diagnosis of SARS-CoV-2 in the three mostly populated islands of the archipelago: Santa Cruz, San Cristobal and Isabela. A total of 1320 mostly asymptomatic, non-hospitalized individuals participated in this surveillance screening, as well as a few cases of suspected COVID-19, and close contacts. Floreana was not included during this first sampling due to a complete island lockdown imposed by its population.
For the second screening, carried out during mid-September 2020, 1160 community-dwelling individuals were tested for SARS-CoV-2 infection by RT-qPCR in the same islands where the first screening was carried out. Furthermore, a total number of 1001 serological tests were applied in the population of the Galapagos Islands, now including all the population of Floreana. This second screening was carried out a few weeks after the travel restrictions to the islands were relaxed and new cases of COVID-19 were detected.
Following the first massive screening in April–May 2020, the islands were declared COVID-19 free, so during the period between the two screenings (June–August), sentinel SARS-CoV-2 testing was carried out only on suspected SARS-CoV-2 cases.
It is important to point out that the selection of individuals to be tested was not random. The local authorities from the Ministry of Health determined the inclusion criteria, and frontline workers such as health staff, airport staff, transportation staff, police and military were prioritized among the asymptomatic individuals included in the study. Additionally, when random household selection was possible, only one adult individual per household was included.
Sample collection, RNA extraction and RT-qPCR for SARS-CoV-2 diagnosis using the CDC protocol
Nasopharyngeal swabs were collected on 0.5 mL TE pH 8 buffer for SARS-CoV-2 diagnosis by RT-qPCR following an adapted version of the Centers for Disease Control and Prevention (CDC) protocol by using PureLink Viral RNA/DNA Mini Kit (Invitrogen, USA) as an alternative RNA extraction method and CFX96 BioRad instrument12-19. Briefly, the CDC designed RT-qPCR FDA EUA 2019-nCoV CDC kit (IDT, USA) is based on N1 and N2 probes to detect SARS-CoV-2 and RNase P as an RNA extraction quality control18,19. Also, negative controls (TE pH 8 buffer) were included as control for carryover contamination, one for each set of RNA extractions, to guarantee that only true positives were reported. For viral load calculation, the 2019-nCoV N-positive control (IDT, USA) was used, provided at 200.000 genome equivalents/μL, and a factor of 200 was applied to convert the viral loads to genome equivalents/mL and then converted to logarithmic scale.
Serological test for anti SARS-CoV-2 IgG
A commercially available lateral-flow immunochromatographic anti-SARS-CoV-2 IgG from INNOVITA (TANGSHAN Biological Technology Co. Ltd, Hebei, China) was used. The appropriate sample volume (20 uL of venous whole blood) was transferred to the indicated sample port, followed immediately by provided diluent, following manufacturer instructions. The lateral flow cartridges were incubated for 15 minutes at room temperature before readings. Result readings were done according to manufacturer instructions by two independent laboratory technicians blinded to specimen status. According to the manufacturer manual, the expected sensitivity and specificity for the test are 87.3% and 100%, respectively. We carried out an internal evaluation with 127 serum samples from SARS-CoV-2 positive individuals by RT-qPCR and 40 serum samples prior to COVID-19 pandemic, and we identified a sensitivity of 79.5% and specificity of 100%20.
Statistical analysis
For the statistical analysis of data, positivity rates were calculated for each island, as well as for different age groups and sexes. Seroprevalence was also calculated for every island. To assess differences in the positivity rates, the χ2 test for comparison of proportions was applied. All statistical analysis was carried out using R software (R-Project; https://cran.r-project.org).
Ethics approval
All participants signed an informed consent to participate freely and voluntarily in the molecular diagnosis of SARS-CoV-2. This study is a secondary analysis of the anonymized laboratory results from a previous surveillance testing done in the context of COVID-19 pandemic. The study was approved by Institutional Review Board from Hospital General San Francisco (Quito) with code CEISH-HGSF-2021-002.
Results
Two massive surveillance samplings were carried out in different islands of the Galapagos archipelago during April–May and September 2020. A total of 2480 individuals of the 25 124 Galapagos inhabitants participated in these two screenings. Overall, 9.87% of the population was tested for SARS-CoV-2 infection, ranging from 7% to 11% depending on the island (Fig1).
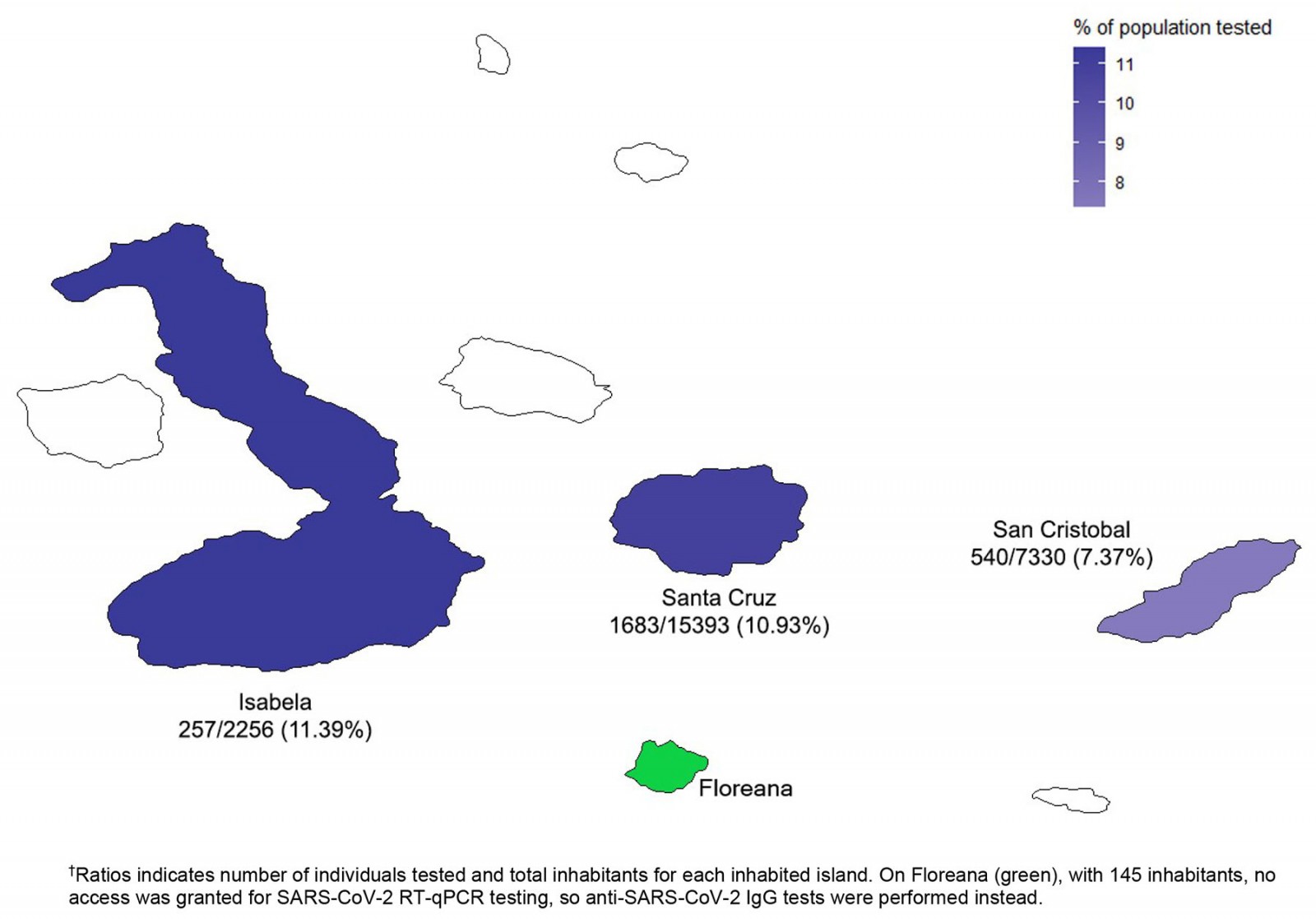 Figure 1: Number of individuals tested for SARS-CoV-2 infection in the Galapagos Islands surveillance.†
Figure 1: Number of individuals tested for SARS-CoV-2 infection in the Galapagos Islands surveillance.†
April–May 2020 SARS-CoV-2 surveillance
During the first sampling, a total of 1320 people were tested for SARS-CoV-2 by RT-qPCR, distributed around three different islands: Isabela, San Cristobal and Santa Cruz (Fig2A). The distribution of the individuals tested by sex and age is detailed in Figures 2B and 2C. There were 865 males and 455 females included in the testing. Most of the individuals were adults aged between 20 and 40 years (mean 36.73±0.37 years). The overall SARS-CoV-2 infection rate was 6.74% (89 SARS-CoV-2 positive individuals, 95%CI 5.51–8.22; Fig2D).
From the subjects who tested positive for SARS-CoV-2, more positive cases were found in males (66/89, 74.16%) than in females (23/89, 25.84%) (Fig2E). The average age for positives cases was 36.5±1.24 years. The SARS-CoV-2 infection rate for males was 7.63% (66/865) and 5.06% (23/455) for females. According to the analysis, no significant difference (p>0.05) was found among those values.
Regarding age groups, different SARS-CoV-2 infection rates were found when comparing children (2/65, 3.08%), young adults (56/768, 7.39%), adults (27/419, 6.44%), and elderly (4/68, 5.88%). Nevertheless, no significant differences neither trends were found among those age groups.
By contrast, there were significant differences in the SARS-CoV-2 infection rate for each island. Isabela had 14 SARS-CoV-2 positive cases out of 170 individuals sampled, an infection rate of 8.24% (95%CI 4.97–13.35). San Cristobal had an infection rate of 1.47% (95%CI 0.63–3.40), with only five positive individuals out of 340 tested. Santa Cruz had 70 SARS-CoV-2 positive individuals out of 810 samples, giving an infection rate of 8.64% (95%CI 6.9–10.78). The main differences in prevalence were found between San Cristobal compared to Isabela and Santa Cruz.
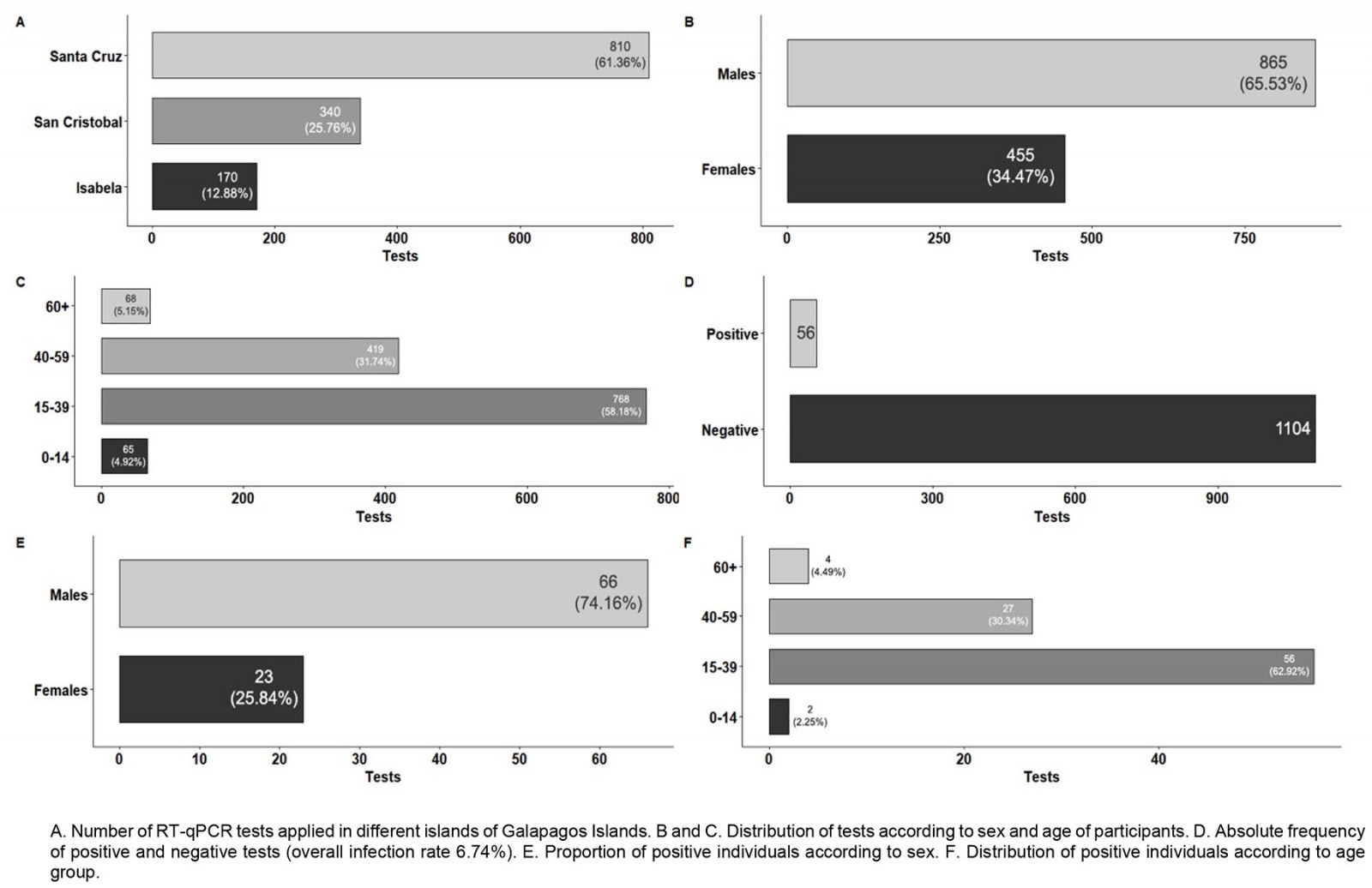 Figure 2: Results from April–May 2020 SARS-CoV-2 surveillance in Galapagos Islands.
Figure 2: Results from April–May 2020 SARS-CoV-2 surveillance in Galapagos Islands.
September 2020 SARS-CoV-2 surveillance
During the second sampling carried out during September 2020, a total number of 1160 individuals (mean age 37.68±0.38 years) were tested for SARS-CoV-2 infection. The distribution of samples among islands was 873 individuals for Santa Cruz, 200 individuals for San Cristobal and 87 individuals for Isabela. The characteristics of the tested population for SARS-CoV-2 RT-qPCR in this second surveillance are detailed in Figure 3. The overall SARS-CoV-2 infection rate found was 4.83% (56 SARS-CoV-2 positive individuals, 95%CI 3.74–6.22; Fig3D). This SARS-CoV-2 infection rate was significantly smaller than the one in April–May 2020 (p<0.05).
For the subjects who tested positive for SARS-CoV-2 in September 2020, more positive cases were found in males (34/56, 60.71%) than in females (22/56, 39.29%), as detailed in Figure 3E. The infection rate for males was 4.41% (34/771), and 5.66% (22/389) for females, with no significant difference for those values (p>0.05). Concerning the different age ranges, children had the highest infection rate (7.69%, 2/26), followed by young adults (5.05%, 35/693), adults (4.58%, 17/371) and elderly people (2.86%, 2/70), although no significant difference for those values was found (p>0.05).
The SARS-CoV-2 infection rates for the different islands were 5.5% for Santa Cruz (95%CI 4.17–7.21), 3.45% for Isabela (95%CI 1.18–9.65) and 2.5% for San Cristobal (95%CI 1.07–5.72), although no significant difference for those values was found (p>0.05).
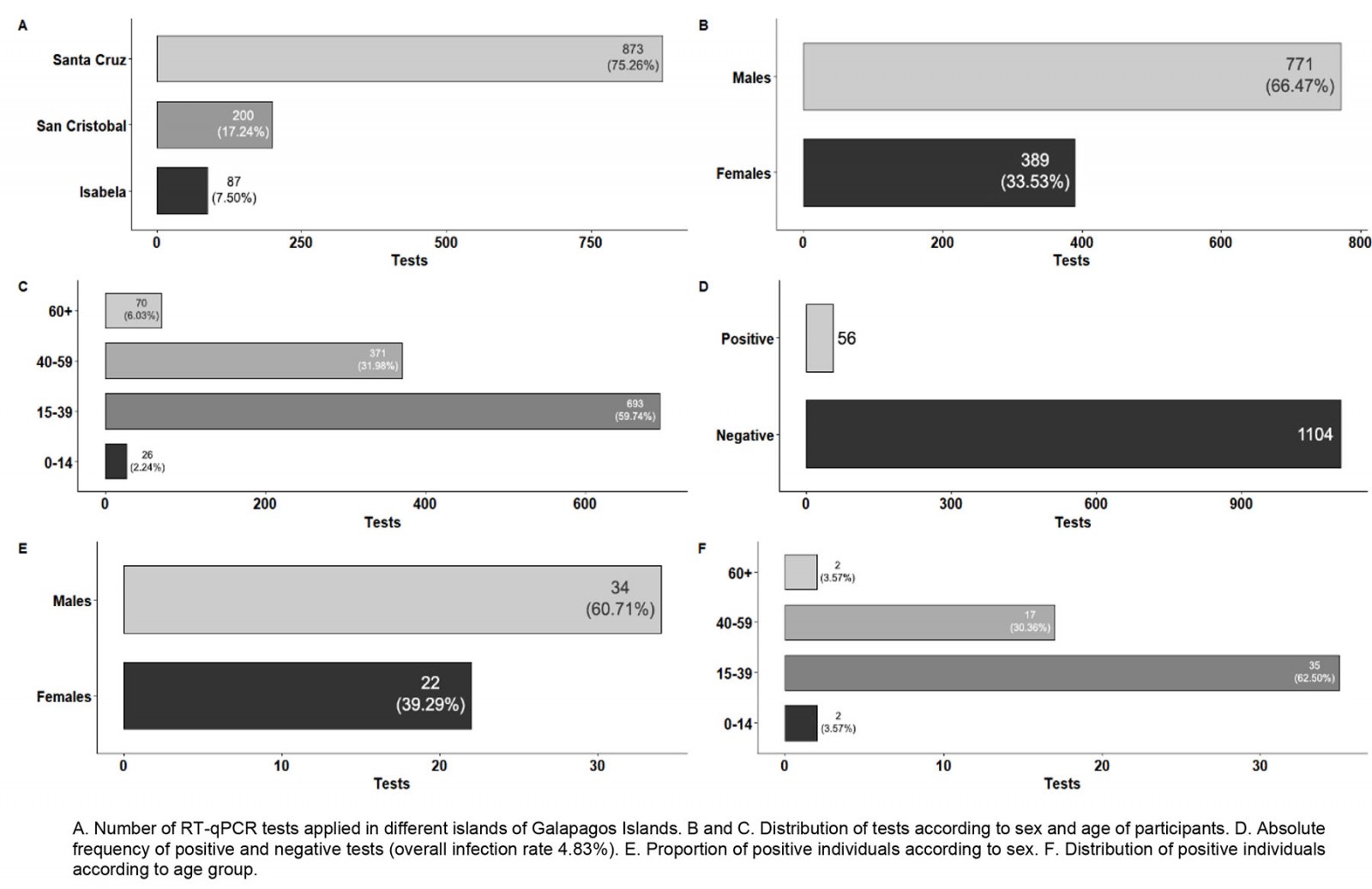 Figure 3: Results from September 2020 SARS-CoV-2 surveillance in Galapagos Islands.
Figure 3: Results from September 2020 SARS-CoV-2 surveillance in Galapagos Islands.
Anti-SARS-CoV-2 IgG seroprevalence in Galapagos Islands by September 2020
During the SARS-CoV-2 surveillance in September 2020, a total number of 1001 individuals were also tested for IgG against SARS-CoV-2 (Fig4). The overall anti-SARS-CoV-2 IgG seroprevalence reached was 3.30% (33 seropositive individuals, 95%CI 2.36–4.59; Fig4D). The seroprevalence values for females and males were 2.56% and 3.70%, although these values are not significantly different. Moreover, no statistically significant differences in seroprevalence were found among age groups or among different islands. The observed seroprevalences for the different islands (Fig5) were 3.36% for Santa Cruz (95%CI 2.27–4.95), 1.15% for Isabela (95%CI 0.20–6.23) and 4.0% for San Cristobal (95%CI 2.04–7.69). The 145 inhabitants of Floreana were tested, and no positive cases for anti-SARS-CoV-2 IgG were found.
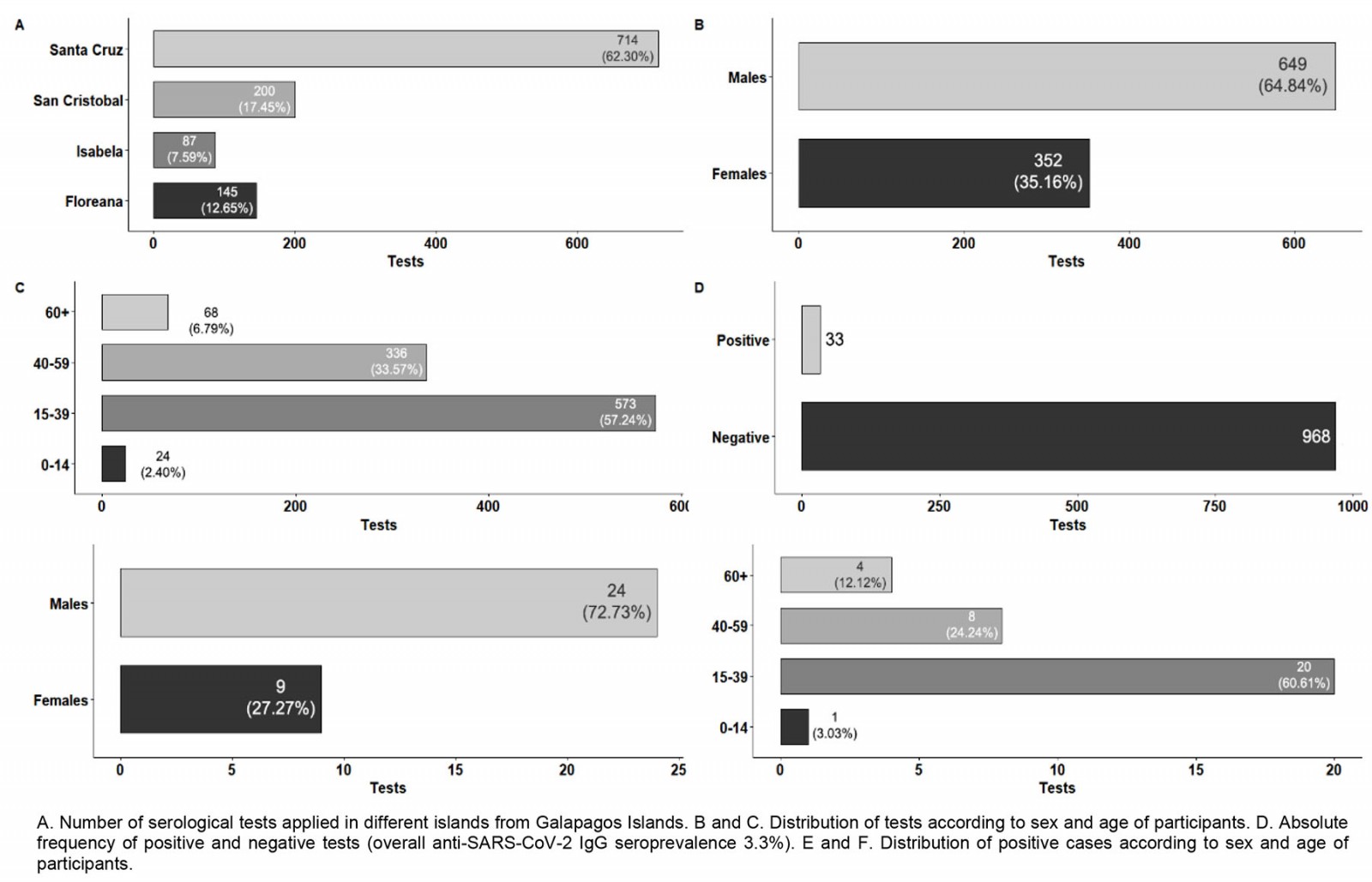 Figure 4: Results for September 2020 anti-SARS-CoV-2 IgG surveillance in Galapagos Islands.
Figure 4: Results for September 2020 anti-SARS-CoV-2 IgG surveillance in Galapagos Islands.
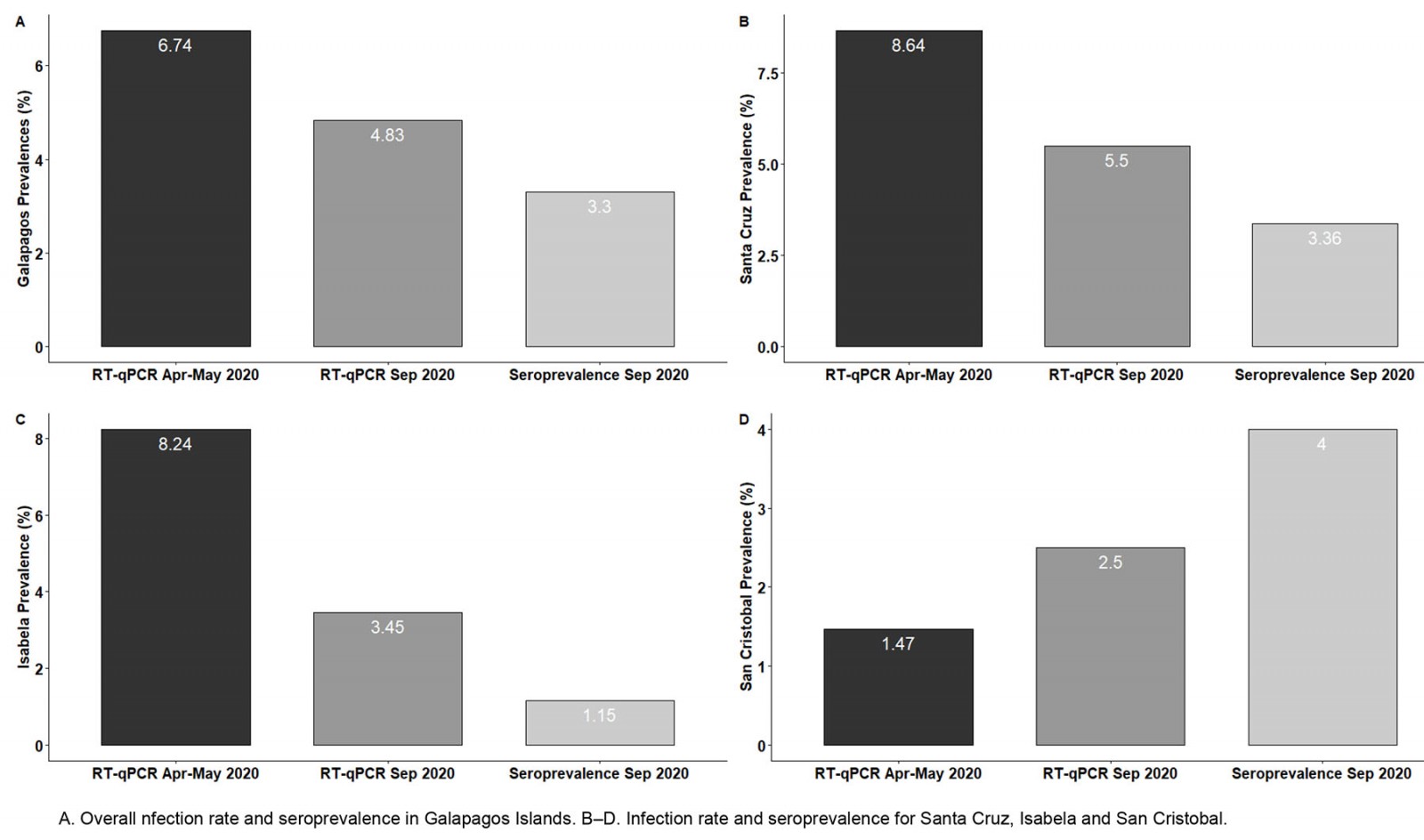 Figure 5: Comparison of SARS-CoV-2 infection rates and anti-SARS-CoV-2 IgG seroprevalence in Galapagos Islands for surveillance interventions during April–May 2020 and September 2020.
Figure 5: Comparison of SARS-CoV-2 infection rates and anti-SARS-CoV-2 IgG seroprevalence in Galapagos Islands for surveillance interventions during April–May 2020 and September 2020.
Viral load distribution among SARS-CoV-2 positive individuals and community-dwelling ‘super spreaders’
During both screenings, the viral loads for positive cases were assessed (Fig6). An important outcome was that 8 out of 89 SARS-CoV-2 positive individuals had viral loads of over 1 × 106 copies/mL during the April–May 2020 surveillance, representing 8.98% of the total positive cases (Fig6A); and 12 out of 56 individuals had viral loads of over 1 × 108 during the September 2020 screening, representing 21.4% of the total positive cases (Fig6B). So, the presence of potential community-dwelling SARS-CoV-2 ‘super spreaders’ was reported during both surveillance interventions.
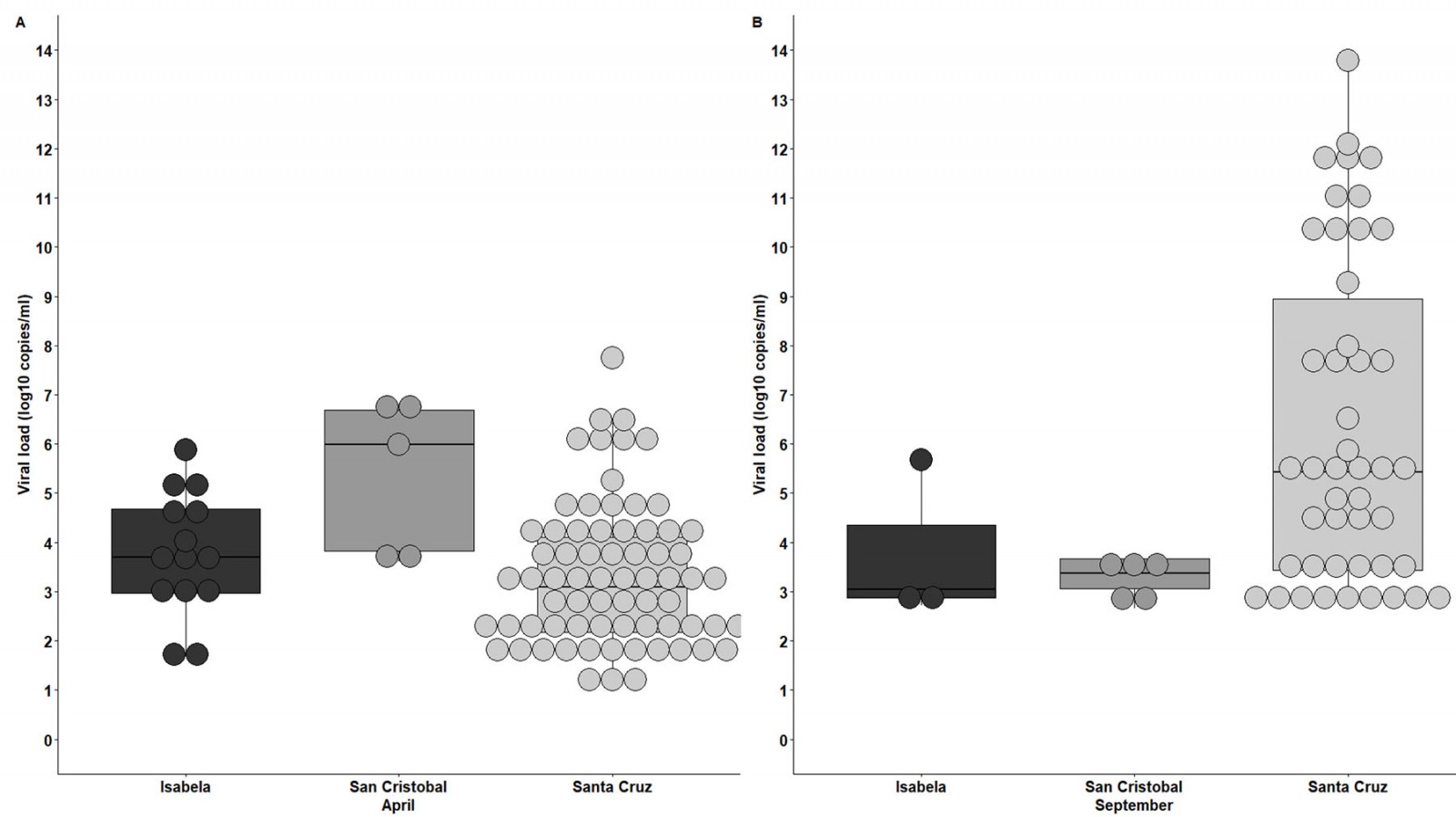 Figure 6: SARS-CoV-2 viral load distribution among positive individuals for each of the inhabited Galapagos Islands for surveillance interventions in April–May 2020 (A) and September 2020 (B).
Figure 6: SARS-CoV-2 viral load distribution among positive individuals for each of the inhabited Galapagos Islands for surveillance interventions in April–May 2020 (A) and September 2020 (B).
Discussion
Epidemiological information for SARS-CoV-2 in South American countries is scarce and mainly based on public health authority reports. These reports are usually not realistic, mainly due to poor SARS-CoV-2 testing capacities and delays in results reporting5,6,21-24. Up to April 2022, less than 3 million SARS-CoV-2 RT-qPCR tests had been done since the COVID-19 outbreak in February 2020, for a population of over 17 million people. The lack of testing capacity affected mostly non-urban populations far from the main cities of Guayaquil, Quito and Cuenca, where the Ministry of Health concentrated the SARS-CoV-2 testing capacities at the laboratory networks of the Instituto Nacional de Salud Publica e Investigación. Despite the efforts from our colleagues from this institution, lack of human resources and proper funding allowed less than 3000 tests daily for the whole country public health system6,21. Unfortunately, this lack of testing capacities did not allow identification of enough cases to slow COVID-19 transmission and avoid the dreadful scenes observed in Guayaquil during the first month of the pandemic, and sustained community transmission for months5-9,23,25-28. In Ecuador, several universities have reported dramatic infection rates in rural communities out of the scope of the hospitals’ focused testing implemented by the Ministry of Health, including extremely remote isolated rural and indigenous communities from the Amazonia, where uncontrolled COVID-19 community transmission was reported from the early stages of the pandemic5-9. So far, those results have confirmed that geographic isolation itself does not protect against SARS-CoV-2 spillover5-9.
In contrast to continental Ecuador, the Galapagos Islands had low COVID-19 prevalence7,26,29-32. By 6 April 2020, Galapagos Islands had the first SARS-CoV-2 detection laboratory within the public health system out of the network of the Instituto Nacional de Salud Pública e Investigación26. This fact allowed SARS-CoV-2 testing on site for this remote province; moreover, Galapagos Islands had a daily testing capacity of 100–200 samples for a population of less than 30 000 people since the first weeks of the COVID-19 outbreak6,26. Under this scenario, the positivity ratios for the islands were less than 10%, suggesting a correct handling of the COVID-19 pandemic compared to the continental Ecuadorian provinces’ positivity ratios of over 25%. This fact was supported and confirmed by our massive surveillance interventions, where infection rates were reduced from 6.74% in April 2020 to 4.83% in September 2020. This also suggests optimal sentinel SARS-CoV-2 testing for handling and isolation of cases during the months between the two massive screenings. Moreover, there was a bias associated with the inclusion criteria for SARS-CoV-2 testing in the Galapagos surveillance intervention: symptomatic individuals and their close contacts, and frontline workers at high risk of SARS-CoV-2 exposure, where prioritized. So, it is highly plausible that the true positivity rate for the Galapagos population was even lower than described, reinforcing the success of this intervention.
When the COVID-19 pandemic numbers for the Galapagos Islands are compared with Ecuador, the importance of onsite high testing capacity to control SARS-CoV-2 spread is confirmed. As we explained in the introduction, this was the only difference in the public health system infrastructure between Galapagos and continental Ecuador, so any potential bias associated with better prevention health policies was ruled out. During the two massive screenings described in this report, 9.87% of Galapagos population was tested within a few weeks. By contrast, by the end of May 2021, more than a year after the COVID-19 outbreak, less than 8% of the Ecuadorian population was tested (Fig7). Massive surveillance screenings could not continue in Galapagos beyond September 2020 due to lack of funding, but the sentinel SARS-CoV-2 testing capacity remained on site. As of May 2021, Galapagos had a much lower positivity ratio (11.59%) than Ecuador (31.15%)5,24. This better COVID-19 epidemiological control in the Galapagos Islands is also confirmed by comparison of mortality ratios with those of Ecuador (Figs7,8). The overall mortality rate for Ecuador was 1.07% when only confirmed deaths from SARS-CoV-2 were taken into account, and 1.46% when cumulative (confirmed and probable) deaths were considered. For the Galapagos Islands, the mortality rate reached 0.08% relative to confirmed deaths and 0.16% relative to cumulative deaths. Furthermore, the Galapagos Islands has the lowest mortality ratio of all Ecuadorian provinces, while provinces like Santa Elena have values greater than 3%, consistent with the severe COVID-19 community transmission outbreaks reported for those locations5,9. Nevertheless, there is also a big difference between the additional deaths and the COVID-19 confirmed deaths for the country, which suggests a shallow data management and report24.
As we have described in the results, several of the SARS-CoV-2 positive individuals had viral loads associated to ‘super spreaders’33. As those were community-dwelling individuals, massive screening of asymptomatic populations should be implemented when possible for a successful COVID-19 surveillance program like the one described here.
Regarding the epidemiological scenario in Floreana, this inhabited island has a substantially smaller population: only 145 individuals. This community chose self-isolation from the other inhabited islands (where airports were available, although operating with restrictions) for several months, and no cases of COVID-19 were reported up to September 2020. So far, no COVID-19 cases have been reported for Floreana Island during the COVID-19 pandemic, making this territory one of the few worldwide remaining COVID-19 free.
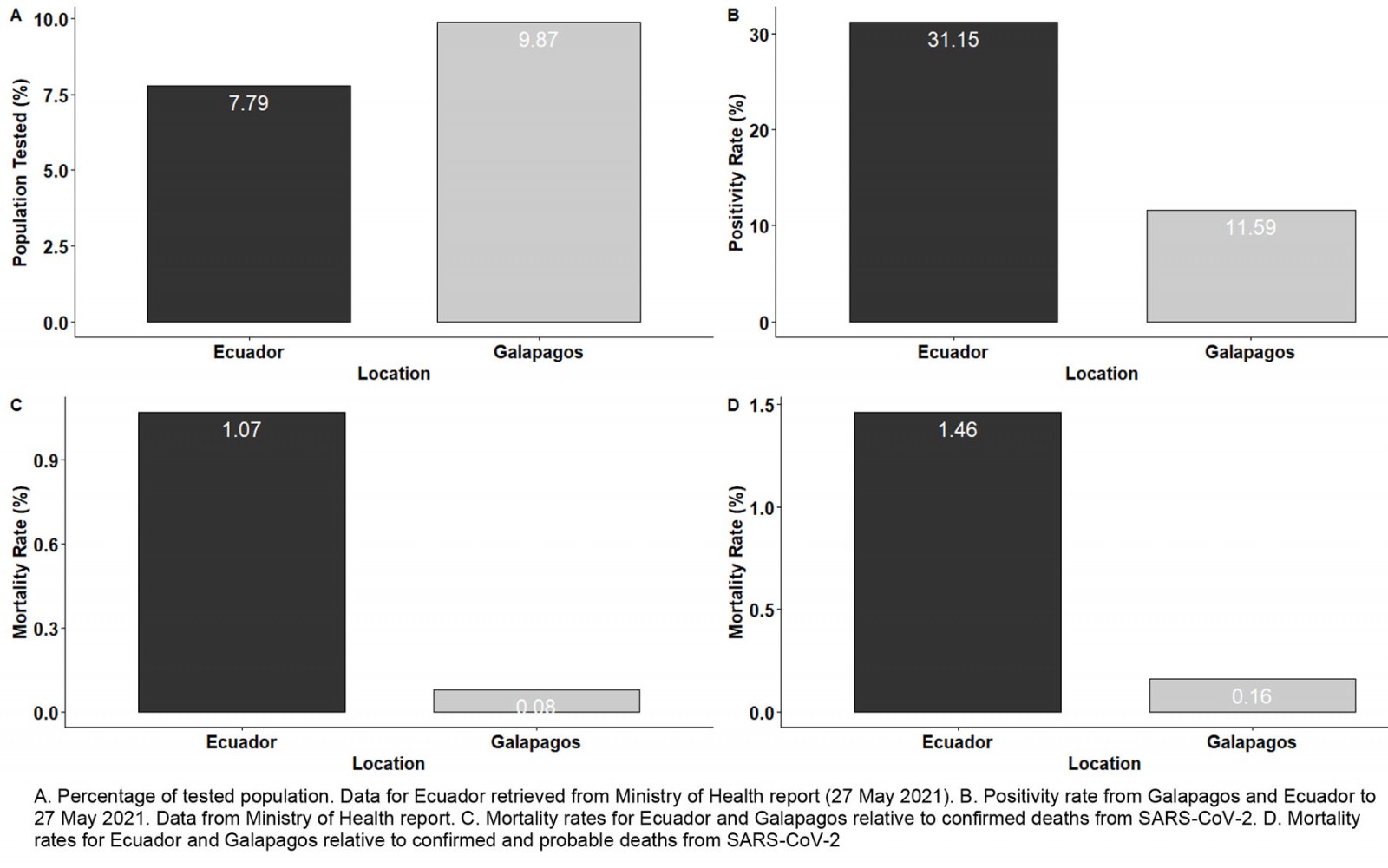 Figure 7: Comparisons of population tested, positivity and mortality rates between the Galapagos Islands and Ecuador.
Figure 7: Comparisons of population tested, positivity and mortality rates between the Galapagos Islands and Ecuador.
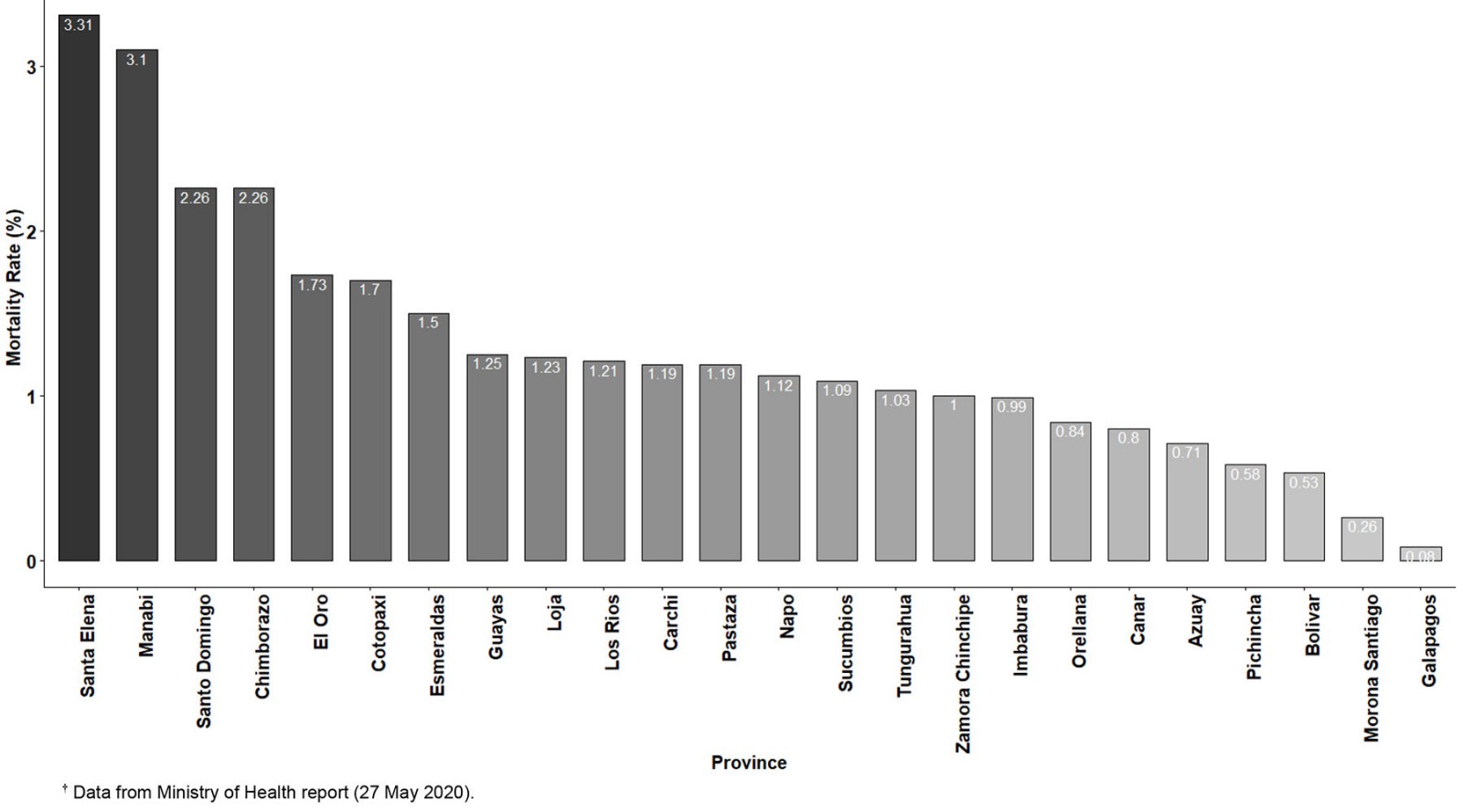 Figure 8: Mortality rates for all Ecuadorian provinces, calculated as confirmed deaths from SARS-CoV-2 infection per total positive RT-qPCR tests performed.†
Figure 8: Mortality rates for all Ecuadorian provinces, calculated as confirmed deaths from SARS-CoV-2 infection per total positive RT-qPCR tests performed.†
Conclusion
Although our study had some limitations, including the lack of access to data for SARS-CoV-2 contact tracing and case tracing for the positives detected in the months following both our screenings, our results confirm the importance of massive testing and implementation of onsite laboratory infrastructure for remote locations to control the spread of SARS-CoV-2 virus. Another plausible explanation for the successful control of COVID-19 outbreak in Galapagos could be associated with geographic location, and the closure of local airports during the early stages of pandemics, followed by a restriction on daily flights after the lockdown was lifted, and the reduction in tourists due to COVID-19 pandemic economic effects worldwide. For instance, similar results have been described for the Faroe Islands34 and island nations of the Caribbean35 and Africa36. However, the Faroe Islands belong to Denmark, a high-income country with a high SARS-CoV-2 testing capacity34. Moreover, the Caribbean and African island nations described in those studies35,36 had SARS-CoV-2 testing facilities on site from the beginning of COVID-19 pandemic, while the Galapagos Islands had to improve a SARS-CoV-2 testing laboratory on site in April 20206,26. Nevertheless, those same studies reported a better containment of SARS-CoV-2 spread for island nations with higher testing capacities35.
In conclusion, although the geographic isolation of Galapagos Islands facilitated COVID-19 control, we could state that the strategy of periodical massive screenings of community dwelling population, as implemented in Galapagos Islands, constituted an essential tool to prevent important SARS-CoV-2 outbreaks and should be considered for future pandemics.







