Introduction
The COVID-19 pandemic dominated the global and Australian healthcare landscape after being declared in March 2020 and was of particularly high public health priority across Queensland after the state borders opened for quarantine-free travel from the rest of Australia on 13 December 20211,2. The first outbreak of COVID-19 in the Torres and Cape region of Far North Queensland was declared in late December 2021.
The Torres and Cape Hospital and Health Service (TCHHS) is one of 16 regional health service organisations within Queensland Health3. It comprises 31 primary healthcare centres and four rural hospitals, servicing a population of approximately 25 000 people across more than 130 000 square kilometres3,4. The region is home to approximately 16 000 Aboriginal and Torres Strait Islander residents (64% of the population) (hereafter respectfully referred to as First Nations peoples)4.
There was considerable concern in Australia that COVID-19 outbreaks would have catastrophic consequences for vulnerable populations living in rural and remote communities with limited access to health services5,6. First Nations peoples continue to experience poorer health outcomes than non-Indigenous Australians, with the Torres and Cape no exception. The burden of disease among First Nations peoples is estimated at 2.2 times that of the broader Australian population and the figure is even higher for those living in remote and very remote communities6,7. Disparities in health services access, social determinants of health and other disease risk factors contribute to the poorer health and wellbeing of First Nations peoples in Australia8,9.
During 2020 and 2021, TCHHS, in partnership with local First Nations councils and health service stakeholders, implemented a series of COVID-19 preparatory measures to minimise potential COVID-19 morbidity and mortality in the region, including the establishment of a COVID-19 public health team, the successful roll-out of a COVID-19 vaccination program, the delivery of a culturally safe public health messaging campaign, and considerable COVID-19 logistics and outbreak planning. Planning included design of the telephone-based COVID-19 Care at Home program, a culturally safe virtual health program that blended public health and clinical care models to support the health and non-health needs of those with COVID-19 and their families throughout mandatory isolation periods. In addition to frequent telephone contact with cases and escalation of clinical concerns, the program centralised the coordination and delivery of COVID-19 therapeutics for eligible cases, aiming to ensure those living in the regional and remote communities of the Torres and Cape region had access to approved COVID-19 treatments in equity with cases in metropolitan areas of Australia.
Approved COVID-19 therapeutics available for this program included one intravenous monoclonal antibody (sotrovimab) and two oral antiviral therapies: nirmatrelvir and ritonavir (Paxlovid®, Pfizer Australia) and molnupiravir (Lagevrio®, Merck Sharp & Dohme Australia), which were all provisionally registered with the Therapeutic Goods Administration (TGA)10-12. Queensland Health prescribing guidelines supported the use of all three therapies in the management of mild to moderate COVID-19 disease, with eligibility criteria for each therapy based around case demographics (age and First Nations status), COVID-19 vaccination status and specific comorbidities known to increase risk of severe COVID-19 disease. Sotrovimab, as a single intravenous dose infused over 30 minutes, was required to be given within 5 days of onset of COVID-19 illness in a healthcare facility with trained nursing staff and medical officer physical oversight13. Paxlovid and Lagevrio were required to be commenced within the first 5 days of COVID-19 illness, and involved taking three or four tablets, twice daily, for 5 days in total. Therapies carried various contraindications and cautions, including that prescribing guidelines were extrapolated consensus recommendations from limited primary study evidence14.
The COVID-19 Care at Home program, including the coordination and delivery of COVID-19 therapeutics, was launched immediately following notification of the first COVID-19 case in the region on 28 December 2021. Here, the authors describe delivery and uptake of the therapeutics program and offer considerations for the delivery of future public health treatment programs in remote settings.
Methods
COVID-19 cases were those testing positive by SARS-CoV-2 polymerase chain reaction (PCR) or rapid antigen test (RAT) within the TCHHS region, with the majority of tests performed by clinical staff at the local primary healthcare centre or hospital fever clinic. A negative RAT was followed up by a PCR if the treating clinician was concerned the RAT may have returned a false positive in initial screening. Cases were followed up by the TCHHS COVID-19 public health team with an initial public health and clinical screening interview on or following the day of notification.
A Microsoft Excel 2016 notification database was established to record COVID-19 treatment eligibility. Automated formulas in the Excel database were used to screen cases potentially eligible for COVID-19 therapies based on case age, COVID-19 vaccination status, immunosuppression status and existing comorbidities. To ensure cases at greater risk of severe disease were identified for early contact during the peak of the outbreak, cases notified from 12 February 2022 and deemed potentially eligible for therapies were triaged into priority risk groups:
- Priority A: on dialysis or immunosuppressed OR <2 COVID-19 vaccinations and aged ≥50 years OR <2 COVID-19 vaccinations plus comorbidities
- Priority B: ≥2 COVID-19 vaccinations and aged ≥50 years OR ≥2 COVID-19 vaccinations plus comorbidities OR <2 COVID-19 vaccinations and aged 35–49 years
- Priority C: ≥2 COVID-19 vaccinations and aged 35–49 years OR any COVID-19 vaccination status and aged 18–34 years.
Cases deemed potentially eligible for treatment after automated screening were medically reviewed using available electronic medical record information to exclude those not eligible for treatment under the prescribing guidelines. Eligible cases were then individually contacted by phone to discuss treatment options. A public health clinician coordinated the prescribing and administration of therapies for each case consenting to treatment in partnership with a multidisciplinary network of pharmacists, nursing staff, health workers, medical officers, operational assistants and logistics support personnel. Treatments were administered from local primary healthcare facilities and regional hospitals.
Cases with a positive test between the 24 December 2021 and 31 March 2022 outbreak period are reported here. Descriptive statistics including counts and proportions were calculated using Microsoft Excel 2016, and Google Maps was used to map treatment locations. Two-sample χ2 tests were performed for comparison between groups using Stata v15.1 (StataCorp; http://www.stata.com), with p-values below 0.05 considered significant.
Ethics approval
Ethics exemption was granted by the Far North Queensland Human Research Ethics Committee under reference EX/2022/QCH/85537 (Mar v2).
Results
A total of 4744 cases were notified during the outbreak period, of which 217 (4.6%) were deemed eligible for treatment after medical review (Table 1). Treatment was offered to 148/217 cases (68.2%), with 90/148 cases (60.8%) declining treatment and 53/148 cases (35.8%) receiving therapeutic treatment for COVID-19. Among these 53 cases, 29 received sotrovimab (54.7%), 20 received Paxlovid (37.7%) and four received Lagevrio (7.5%) (Figure 2). Oral antiviral treatment was ceased for 2/24 cases (8.3%) who reported adverse reactions to either Paxlovid or Lagevrio.
First Nations peoples accounted for 186/217 cases (85.7%) who were eligible for treatment and 48/53 cases (90.6%) who received treatment. While a greater proportion of notified First Nations cases were eligible for treatment than non-Indigenous cases (5.3%, 2.5%, p<0.001), similar proportions of First Nations and non-Indigenous cases were offered treatment (66.7%, 77.4%, p=0.24). Although not significant, treatment uptake among First Nations cases was almost twice as frequent as uptake among non-Indigenous cases (38.7%, 20.8%, p=0.09).
After the priority triage system was introduced, 59/198 cases (29.8%) were classified as Priority A, 105/198 cases (53.0%) were classified as Priority B and 34/198 cases (17.2%) were classified as Priority C. Of cases offered treatment, a greater proportion of Priority A cases were treated (21/47, 44.7%) than Priority B cases (21/61, 34.4%) or Priority C cases (4/19, 21.1%), although the differences were not statistically significant.
COVID-19 therapeutics were delivered to cases in 16 remote communities during the outbreak period, including nine Cape York communities, five Torres Strait Island communities and two Northern Peninsula Area communities (Fig2). A range of transport was utilised to transfer medications across the region including light aircraft, helicopters, ferries, taxis and local ambulance services. When necessary, charter helicopters were used to transfer medical officers to remote communities or transfer cases to the closest regional hospital for sotrovimab infusion.
Table 1: Cases eligible for, offered and accepting treatment, by First Nations status and sex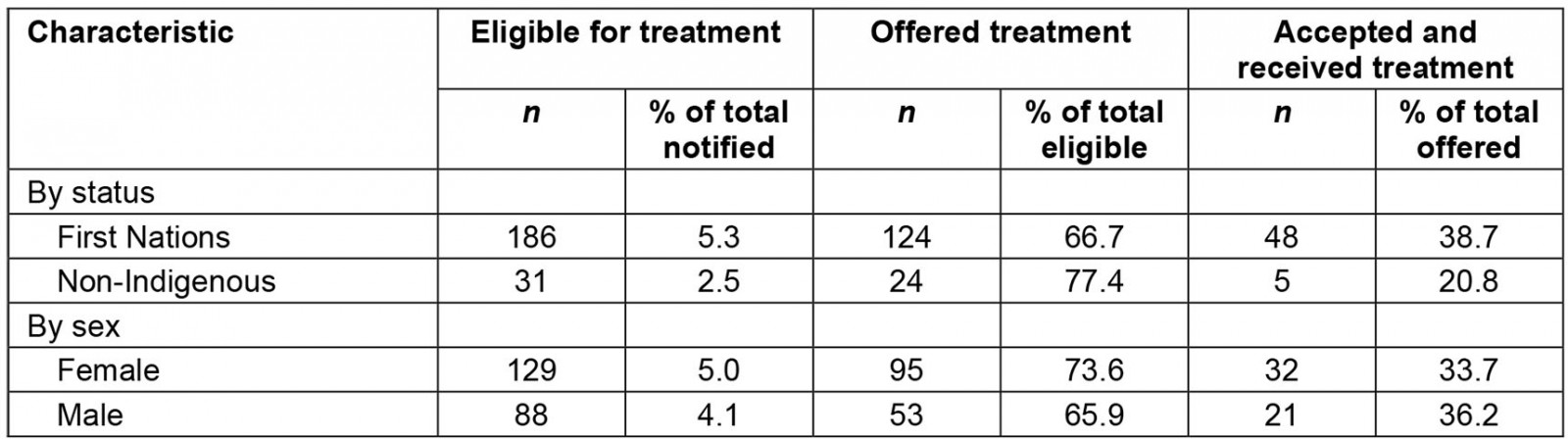
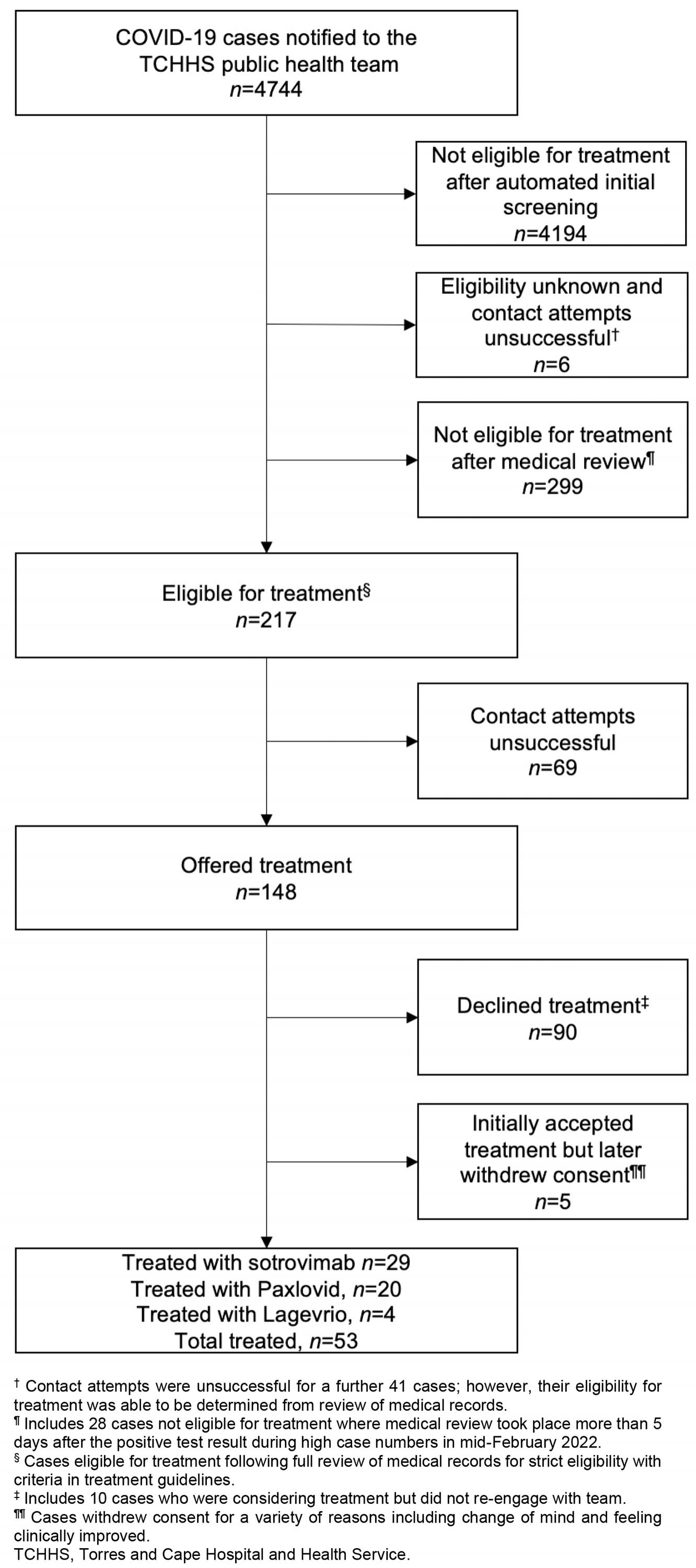 Figure 1: COVID-19 therapeutics flowchart for cases notified December 2021 to March 2022, Torres and Cape Hospital and Health Service.
Figure 1: COVID-19 therapeutics flowchart for cases notified December 2021 to March 2022, Torres and Cape Hospital and Health Service.
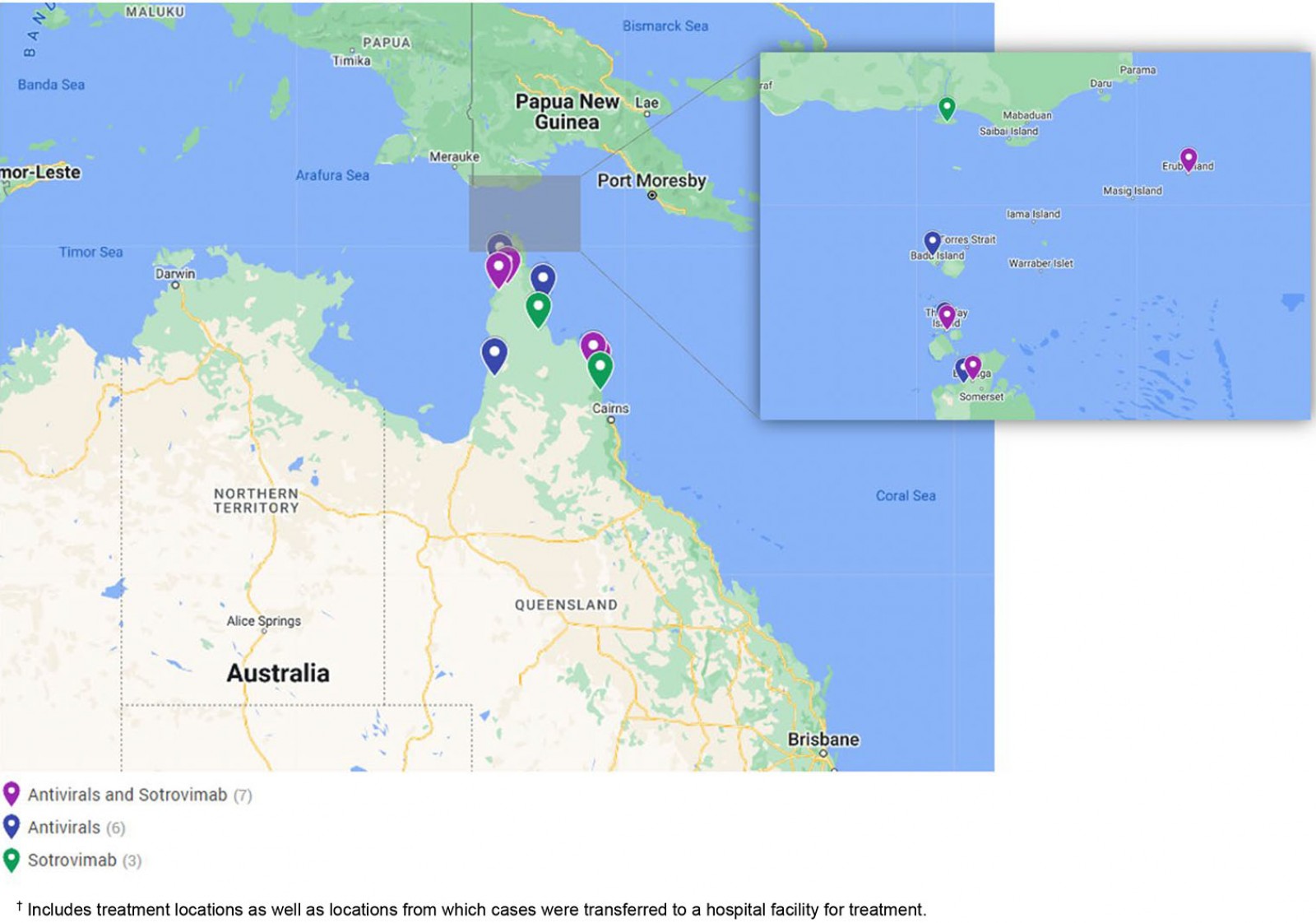 Figure 2: COVID-19 therapeutic treatment locations, December 2021 to March 2022, Torres and Cape Hospital and Health Service.†
Figure 2: COVID-19 therapeutic treatment locations, December 2021 to March 2022, Torres and Cape Hospital and Health Service.†
Discussion
This program demonstrated a successful, centralised, blended public health and primary care model enabling equitable access to COVID-19 therapeutics during the first wave of the omicron variant in the COVID-19 pandemic across the remote Torres and Cape communities in Far North Queensland. Although only 35.8% of those offered therapy accepted treatment, 68.2% of eligible cases were contacted and provided with culturally sensitive, patient-centred education and support in their decision making.
The COVID-19 pandemic has unequivocally highlighted health disparities experienced by vulnerable populations globally, including people with lower socioeconomic status, migrants and minorities15. Early in the pandemic, attention was drawn to First Nations communities across Australia and globally, and the significant risks the pandemic could have on their health and culture unless appropriate health resources and support were provided6. The Australian Government acknowledged this priority and established the National Partnership Agreement to financially support the COVID-19 outbreak response across Australia16. This funding facilitated a local health service COVID-19 outbreak response and supported equity of therapeutic access for First Nations communities in remote Far North Queensland that may not have been otherwise feasible.
The coordination of care by public health clinicians provided the advantages of broad outbreak oversight and currency with the rapidly evolving COVID-19 treatment guidelines, which were then communicated to and coordinated with primary healthcare professionals on the ground. This model leveraged partnerships with local primary healthcare centres and hospital staff to coordinate all treatment options available to cases, which ranged from hospital-based intravenous infusions to at-home oral medications. This provided consistent, reliable health policies, procedures and systems to the health service at a time where there was significant national hesitancy around treatment safety and efficacy. This approach differed from the mainstream approach in Australia, which relied on healthcare providers in hospitals and primary care settings to individually identify eligible cases and remain abreast of large volumes of rapidly evolving health information and changes in prescribing guidelines. This mainstream approach raised some concerns, with reports that many eligible cases were not provided with timely access to COVID-19 therapies, despite residing in metropolitan or regional areas17.
To date, the burden of severe COVID-19 disease across the Torres and Cape region has been substantially lower than predicted (unpublished data). Clinically, COVID-19 therapeutics likely played an insignificant role in this outcome, given the relatively small proportion of notified cases receiving treatment (1.1%, 53/4744), and the relatively small (though significant, in unimmunised and at-risk patients) reduction in hospitalisations and death outcomes with treatment in even the primary published data for these medication treatments18-20.
The COVID-19 Care at Home program was not only effective, but also endeavoured to be culturally safe for First Nations people across TCHHS. This was demonstrated by similar rates of uptake of treatment by First Nations and non-Indigenous cases. The program utilised a heterogenous network of public health team and local health service staff, with excellent collective working knowledge of local communities, and frequently involved formal cultural supports such as local Aboriginal or Torres Strait Islander Health Workers. The program also featured flexibility to involve an individual’s familiar care network, for example having linking conversations between a remote patient’s local clinic nurse, base hospital specialist teams, and family or carers.
Prior literature has demonstrated the synergistic effects of a collaborative public health and primary care approach21-23. The public health outbreak response described here builds upon such evidence and demonstrates the potential to employ similar, centrally coordinated models of care to manage other diseases of public health importance across the region, such as rheumatic heart disease and chronic hepatitis B. There are significant challenges to providing essential acute and primary healthcare services in remote areas, not least of which is attracting and retaining healthcare staff. A centralised model can provide quality data and systems support to stretched local healthcare staff, improving the equity and efficiency of primary healthcare delivery, and ultimately contribute to improvements in the health of communities in this region.
There were several challenges for this program. Prescribing guidelines for COVID-19 were altered several times throughout the outbreak period, creating complexity in determining case eligibility and influencing the supply of therapeutics. While sotrovimab was listed in Queensland Health guidelines from 17 December 2021, just prior to the outbreak, oral antiviral medications were not added until 11 February 2022 which was during the peak of local case numbers14. Moreover, medication adherence for antivirals was not captured in this study.
While beyond the scope of this study, it is important to acknowledge that the logistics and cost involved in delivering health care across remote communities is significant. This program facilitated several transfers of medical officers on charter helicopters to remote locations to deliver sotrovimab infusions, and some COVID-19 cases were transported to their nearest hospital by charter aircraft for treatment. Contact attempts for 69 of 217 cases were unsuccessful, contributed to by varying telephone connectivity across remote locations. Improving case contact in future outbreaks could include employing satellite teams based in community to provide house visits to cases.
Conclusion
The COVID-19 Care at Home program demonstrated a novel, public health led approach to delivering time-critical medications to individuals across a large, remote and logistically complex region. The application of similar models to outbreaks and chronic conditions of public health importance offers the potential to address many health access inequities experienced by remote Australian First Nations communities.
Acknowledgements
The authors would like to thank Ivana Gibson for her generous review of this manuscript. We wish to acknowledge the collaborations with and support of all the Torres and Cape Hospital and Health staff who contributed to the management of the first COVID-19 outbreak across the region and the community for their participation in this program.
References
You might also be interested in:
2016 - Mental health nurses in South Africa's public rural primary care settings: a human resource crisis
2005 - Recruiting undergraduates to rural practice: what the students can tell us


