Introduction
Prior research has demonstrated extensive physical health disparities across the lifespan affecting sexual minority (SM) populations1-3. Data from the US National Epidemiologic Survey on Alcohol and Related Conditions demonstrated that sexual minorities are at increased risk for a number of chronic diseases, including gastrointestinal conditions, arthritis, and cirrhosis of the liver, and were more likely to experience a higher number of comorbid chronic disease4. Other studies have replicated these findings and indicate that sexual minorities are also at high risk for cardiovascular disease and stroke compared to heterosexuals3,5-7, and may be so from a much younger age than previously thought8. These disparities may be attributable to elevated rates of C-reactive protein – a biomarker of systemic inflammation associated with increased risk of cardiovascular disease9,10, diabetes11 and cancer12 – among SM populations3,13,14. Although individual risk factors have been implicated in these disparities affecting SM populations, particularly with regards to stigma15-17 and substance use18-21, structural factors may confer additional risk.
Previous work has demonstrated the rate of accumulation of chronic disease burden among rural versus urban populations over a 5-year period, noting that rural populations have a consistently higher burden over time. Not only this, but the disparity between rural and urban populations continues to grow over the study period, a set of findings that is consistent across studies22-26. For example, in a secondary analysis of longitudinal cohort data, rural populations had a higher burden of disease at baseline as well as an increased rate of disease accumulation over time25.
Within rural populations, rural sexual minorities face substantial barriers to care as a result of persistent internalized stigma in combination with stigma from care providers. For example, two recent studies have both noted that rural men who have sex with men are less likely to be engaged in healthcare settings as a result of internalized homophobia and feelings of social exclusion27,28. These issues only become exacerbated as sexual minorities age into older adulthood and face not only sexual minority-specific discrimination (eg discrimination in long-term care facilities)29,30 but also face microaggressions that denigrate all older adults as incapable31-33. These issues are compounded by rural care providers who continue to be among those most likely to hold unfavorable attitudes towards sexual minority populations, although these attitudes continue to improve as more sexual minority-specific training is provided34.
The disparities in physical health experienced by both rural and SM populations suggest that SM individuals who live in rural areas may be at elevated risk for poor physical health as a result of the confluence of their dual minority statuses. Past research supports this, indicating that rural SM residents are at increased risk for negative health outcomes compared to urban-dwelling SM35-37. However, research has yet to use population-based data to assess whether or not disparities affecting rural SMs are larger than those affecting urban SMs. However, this is necessary for informing future public health interventions that aim to reduce substance use and substance-use-associated chronic diseases among sexual minorities in rural and urban areas.
To this address this gap in the literature, we used data from the National Survey on Drug Use and Health (NSDUH) 2015–2019 to assess differences in chronic disease between rural- and urban-dwelling populations. More specifically, we assessed the relationship between sexual identity and each of the following chronic diseases: asthma, high blood pressure, cancer, chronic obstructive pulmonary disease (COPD), diabetes, hepatitis B and C, heart disease, and kidney disease. We also examined differences in these relationships based on sex.
Methods
Study population
Data utilized in this analysis come from the NSDUH datasets, a publicly available, nationally representative cross-sectional survey of private, non-institutionalized US citizens across all 50 states and the District of Columbia38. This is an annual survey that provides information on a host of health-related factors, including health conditions, tobacco, alcohol, and drug use, and substance-use-treatment utilization. Survey sample weights are provided by NSDUH as part of the publicly available data and are used to obtain non-biased estimates for survey outcomes.
The data selected for these analyses were limited to NSDUH data for the years 2015 to 2019. These were selected because 2015 was the first year in which NSDUH assessed self-reported participant sexual identity and 2019 was the final year of data available before the COVID pandemic. Although 2020 data were available, several changes were made to the survey that limited consistency and thus are not included here. The analytic sample was limited to adults, as minors participating in the survey did not receive the set of questions related to sexual identity. Finally, data were aggregated across all study years 2015–2019 (N=210 392) and models were adjusted for year of data collection to ensure consideration of year-specific differences.
Demographic measures
Survey participants self-reported demographic information including age, sex, sexual identity, race and ethnicity, and yearly income. To prevent potential identification of participants, age was provided only as a categorical variable in the publicly available NSDUH dataset: 18–25, 26–34, 35–49, and 50 years or older. As discussed elsewhere39 NSDUH includes a single item representing sex/gender, with two options: ‘male’ and ‘female’. In NSDUH codebooks, there is a lack of clarity regarding whether sex and/or gender are assessed via this item and how this item is administered (self or interviewer reported). This item also does not capture transgender and non-binary identities. Here, we use the term ‘sex’ to refer to the construct this item assesses and the options as female and male. Similarly, race and ethnicity are combined as a single variable: non-Hispanic white, non-Hispanic Black, non-Hispanic Native American/Alaska Native, non-Hispanic Native Hawaiian/Pacific Islander, non-Hispanic Asian, and non-Hispanic Multiracial. Participants reporting a Hispanic ethnicity were coded as such, regardless of their racial identity. Sexual identity was asked as, ‘Which of the following do you consider yourself to be?’, and coded as ‘heterosexual’, ‘lesbian/gay’, or bisexual, the only options provided in the NSDUH dataset. Income was similarly self-reported at the time of interview and coded as yearly income (US$) of <$20,000, $20,000–49,999, $50,000–74,999, and $75,000 or higher.
Rural/urban status
In keeping with the few past studies that have assessed rural versus urban differences using NSDUH data40 we utilized the trichotomous variable of residence in a Core Based Statistical Area (CBSA). Options for this variable were coded as large CBSA (>1 million persons), small CBSA (<1 million persons), or ‘participant does not reside in a CBSA’ (rural). Suburban areas are included in both large and small CBSAs depending on the population size. Due to limitations of power in this analysis, we operationalized this as a dichotomous variable: urban (resides in a CBSA) or rural (does not reside in a CBSA).
Health-related outcomes
Chronic disease was assessed across several health conditions in the dataset including asthma, high blood pressure, history of cancer, COPD, cirrhosis, diabetes, hepatitis B or C, heart disease, kidney disease, and STI status. For each condition, participants were asked whether they were ever told by a doctor or other healthcare professional that they ever had the condition, each variable was operationalized as a dichotomous ‘no’ or ‘yes’ variable. For accuracy, we list cirrhosis here as it is available in the NSDUH dataset; however, too few sexual minorities endorsed this item thus it is not included in our analyses. Although past-year prevalence of each condition was available, these items were quite limited in terms of power thus we chose to include only lifetime prevalence of each disease.
Recent STI status was self-reported and asked in the following manner: ‘During the past 12 months, did you have a sexually transmitted disease such as chlamydia, gonorrhea, herpes, or syphilis?’ The variable did not distinguish between specific STIs and was thus operationalized as a dichotomous variable; it was also only available for the previous 12 months. Lastly, possession of any health insurance at the time of interview (eg private insurance, Medicare, Medicaid, Tricare) was operationalized as a dichotomous variable.
Statistical analyses
Participant characteristics were described using means, standard deviations, and proportions, as appropriate. Multivariable logistic regression models were utilized to assess the association between sexual identity and each of the chronic diseases, adjusting for demographic characteristics, insurance status, and year of data collection. All models were first stratified by rural versus urban status. Secondarily, models were stratified by sex to account for well-documented differences in chronic disease between these populations. All models were weighted to account for NSDUH’s stratified cluster sampling design. Statistical significance was established at α<0.05. All analyses were performed in Stata v17.0 (StataCorp; http://www.stata.com).
Ethics approval
All data are de-identified and publicly available, thus are exempted from institutional review board review.
Results
Among 210 392 participants in the analytic sample across the 5-year study period (Table 1), 22 790 (8.1%) reported residing in a rural environment while 259 978 (91.9%) reported residing in an urban environment. Regarding sexual identity, 195 385 (92.9%) identified as heterosexual, 4640 (2.2%) as gay or lesbian, and 10 367 (4.9%) as bisexual. A plurality of the sample was 35–49 years (n=55 369, 26.3%), identified as non-Hispanic White (n=127 556, 60.6%), reported income of ≥$75,000 (n=70 244, 33.4%), and had health insurance at the time of survey completion (n = 184 996, 88.5%). The number of people participating in the survey each year remained stable across all years at a mean of 42 078 (20%) per year.
Prevalence estimates (Table 2) were combined across all years included in the analytic dataset, 2015–2019. In descending order of lifetime prevalence, 25 921 (12.4%) participants reported high blood pressure, 22 973 (11.0%) reported asthma, 14 302 (6.8%) reported diabetes, 14 002 (6.7%) reported heart disease, 2116 (2.9%) reported COPD, 5471 (2.6%) reported any STI in the past 12 months, 2638 (1.3%) reported lifetime kidney disease, 1910 (0.9%) reported hepatitis B or C, and 1466 (0.7%) reported any form of cancer. Prevalence of disease among females occurred in an identical descending order while prevalence among males swapped prevalence of diabetes for a slightly higher rate of heart disease. Significant differences between females and males existed across all diseases with the sole exception of any history of cancer.
Table 3 presents weighted logistic regression models among females, examining the relationship between sexual identity and each of the health-associated outcomes, stratified by rural versus urban residence. Compared to urban heterosexual females, urban bisexual females had significantly lower odds of having any health insurance (adjusted odds ratio (aOR) 0.88; 95%CI 0.79–0.98). Across health conditions, urban lesbian females had significantly higher odds of asthma (aOR 1.46; 95%CI 1.23–1.74), COPD (aOR 1.63; 95%CI 1.19–2.25), and hepatitis B or C (aOR 2.18; 95%CI 1.28–3.73). Urban bisexual females, compared to urban heterosexual females, had higher odds of asthma (aOR 1.49; 95% CI 1.36–1.63), COPD (aOR 1.87; 95%CI 1.54–2.27), hepatitis B or C (aOR 2.42; 95%CI 1.77–3.31), any heart disease (aOR 1.44; 95%CI 1.21–1.71), and any STI in the previous 12 months (aOR 1.94; 95%CI 1.67–2.24).
Among rural female residents, compared to rural heterosexual females, rural lesbian females reported significantly lower odds of having insurance (aOR 0.36; 95%CI 0.20–0.65). They also had lower odds of COPD (aOR 0.20; 95%CI 0.05–0.74) and diabetes (aOR 0.21; 95%CI 0.05–0.96); however, these confidence intervals are quite wide, suggesting that these odds ratios are based on a small number of rural lesbian females with COPD and diabetes and thus may not replicate. Rural bisexual females, compared to their heterosexual counterparts, had significantly higher odds of asthma (aOR 2.12; 95%CI 1.51–2.99), COPD (aOR 4.03; 95%CI 2.36–6.87), hepatitis B or C (aOR 12.14; 95%CI 4.30–34.31), and any STI in the previous 12 months (aOR 2.94; 95%CI 1.77–4.89). They also had significantly lower odds of cancer (aOR 0.08; 95%CI 0.01–0.64) and kidney disease (aOR 0.21; 95%CI 0.06–0.70); however, confidence intervals were quite wide for these estimates, suggesting that they are based on a small number of bisexual females with these diseases and thus may not replicate.
Table 4 presents similar weighted logistic regression models among males, examining the relationship between sexual identity and each of the health-associated outcomes, stratified by rural versus urban residence. Among urban residents, gay males, compared to heterosexual males, had significantly higher odds of having health insurance (aOR 1.33; 95%CI 1.08–1.63). Urban gay men also had higher odds of asthma (aOR 1.46; 95%CI 1.19–1.80), high blood pressure (aOR 1.26; 95%CI 1.03–1.53), hepatitis B or C (aOR 5.71; 95%CI 4.10–7.95), and any STI in the previous 12 months (aOR 5.84; 95%CI 4.68–7.29). Urban bisexual males, compared to their urban heterosexual counterparts, had significantly higher odds of asthma (aOR 1.53; 95%CI 1.27–1.85), high blood pressure (aOR 1.29; 95%CI 1.03–1.62), COPD (aOR 1.55; 95%CI 1.07–2.27), hepatitis B or C (aOR 2.17; 95%CI 1.23–3.85), and any STI in the previous 12 months (aOR 2.78; 95%CI 2.00–3.86). Among rural male residents, bisexuals had significantly increased odds of high blood pressure (aOR 2.18; 95%CI 1.00–4.76), compared to heterosexuals.
Table 1: Demographic characteristics of participants in the analytic sample, stratified by sex, National Survey on Drug Use and Health, 2015–2019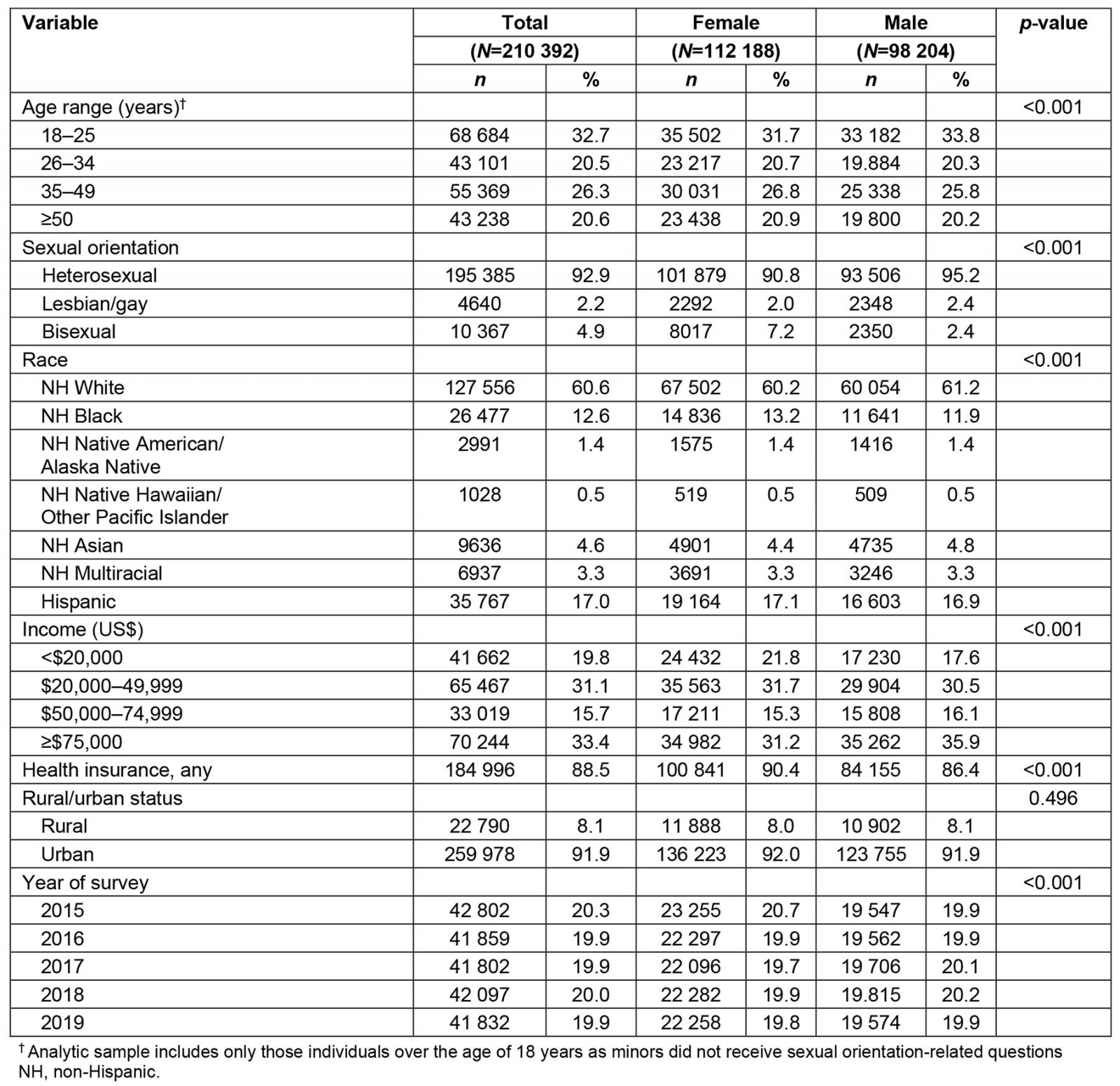
Table 2: Prevalence of chronic disease in the analytic sample, stratified by sex, National Survey on Drug Use and Health, 2015–2019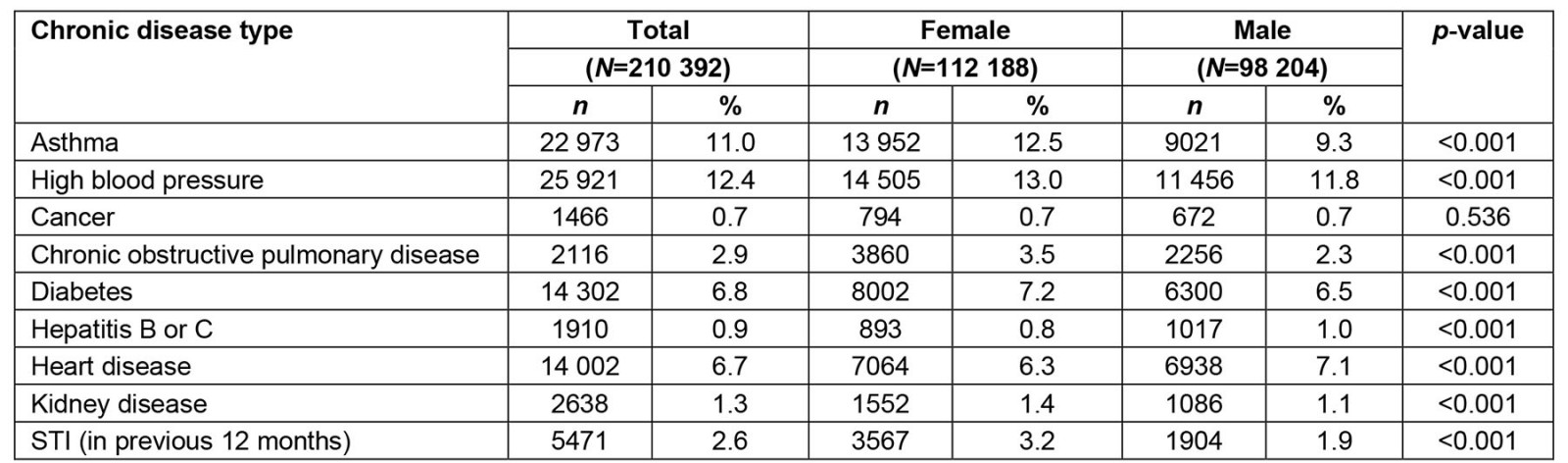
Table 3: Among female participants, multivariable survey-weighted logistic regression analyses† examining the association between sexual minority status and health-associated outcomes, National Survey on Drug Use and Health, 2015–2019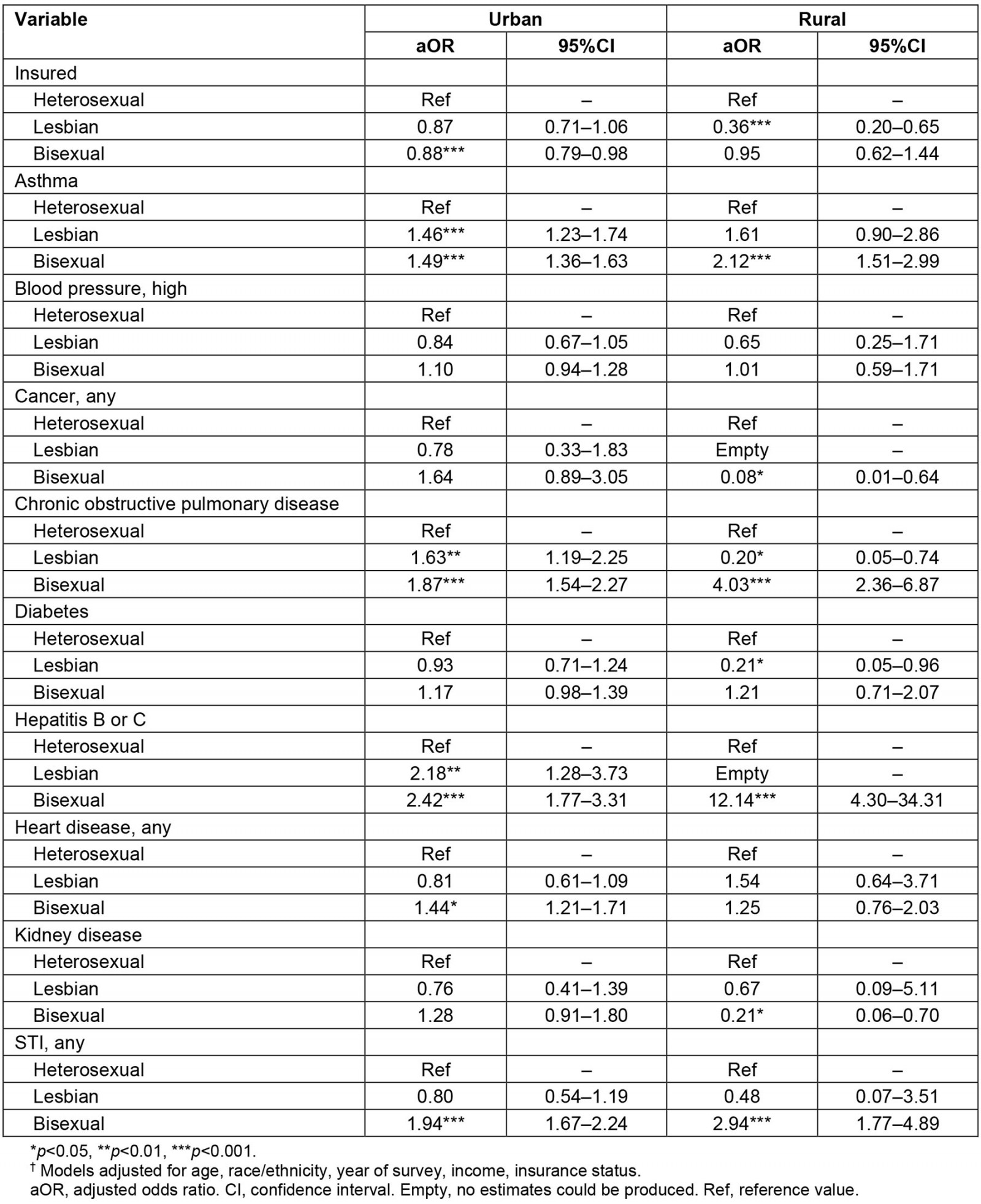
Table 4: Among male participants, multivariable survey-weighted logistic regression analyses† examining the association between sexual minority status and health-associated outcomes, National Survey on Drug Use and Health, 2015–2019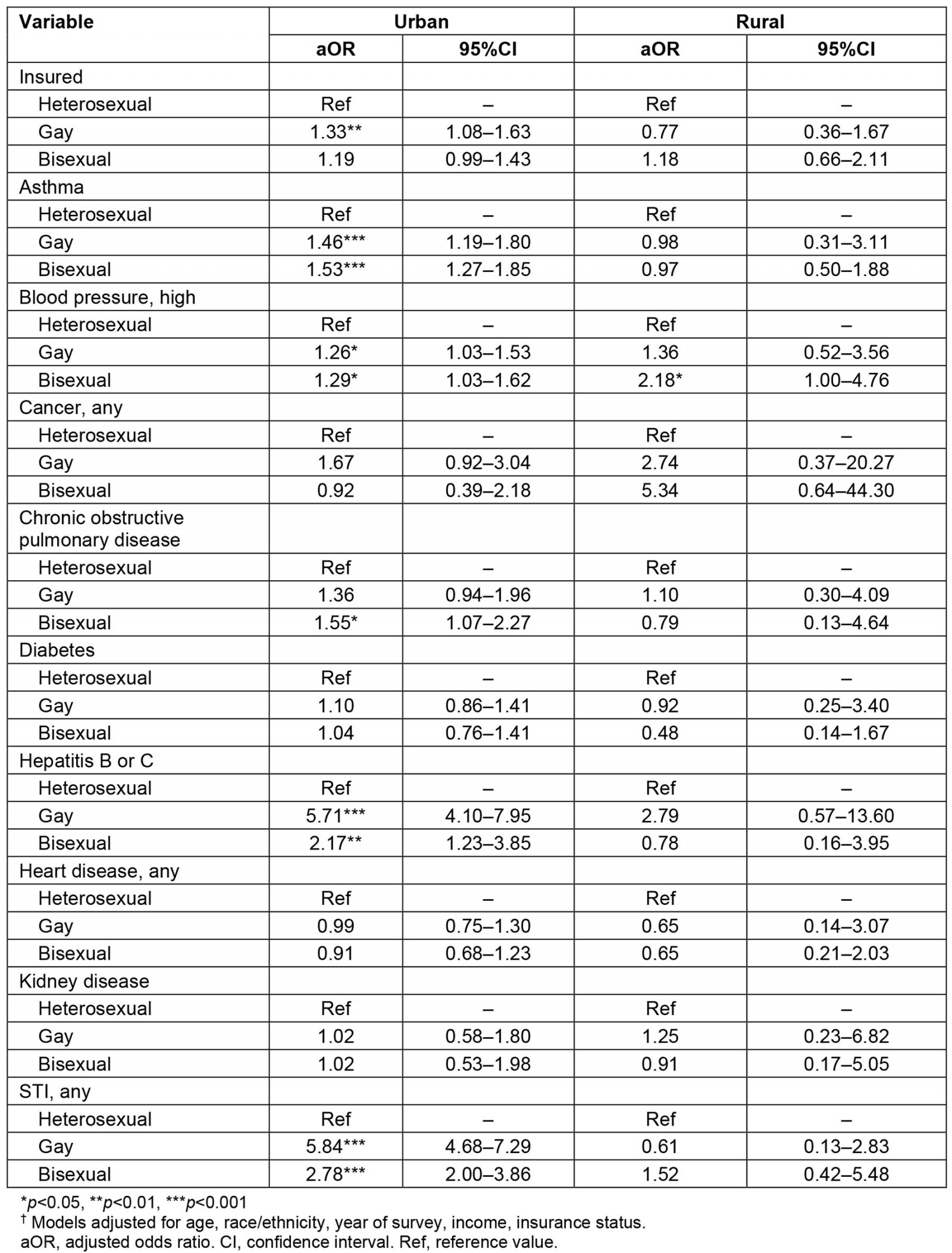
Discussion
Using 2015–2019 data from the representative NSDUH, we assessed whether the likelihood of having insurance and chronic diseases differed by sexual identity based on location of residence in a rural or urban setting. We demonstrated that urban-dwelling gay males were more likely than their heterosexual counterparts to report currently having health insurance. Conversely, urban-dwelling bisexual females were less likely than urban heterosexual females to currently possess health insurance while rural-dwelling lesbian females reported even lower likelihood than those dwelling in urban locations. Higher odds of several chronic diseases were observed among urban gay males (asthma, high blood pressure, hepatitis B or C, and STIs) and urban bisexual males (asthma, COPD, hepatitis B or C, and STIs) compared to urban heterosexuals while fewer differences were observed among rural males. Elevated likelihood of chronic disease was also observed among urban lesbian females (asthma, COPD, and hepatitis B or C) while rural lesbian females experienced no elevated likelihood of chronic disease. Among urban bisexual females, however, higher odds of disease were observed for asthma, COPD, hepatitis B or C, and STIs; odds of each these diseases increased further for rural-dwelling bisexual females. In sum, there are key differences between urban and rural SM populations, and future research should refrain from treating SM populations as homogeneous.
This article adds to a growing body of literature on health disparities among SM populations in several meaningful ways, the first of which is regarding health insurance status. Two key past studies in this area each used data from the National Health Interview Survey (NHIS) to examine changes in insurance status among this population. The first assessed data during the period 2013–2015 and noted that prior low rates of insurance among sexual minorities had largely been eliminated but that issues regarding quality of care persisted41,42. The latter finding is supported by more recent research using data from the National Survey of Family Growth, which demonstrates that SM women are less likely to receive proper reproductive and sexual care counseling in clinical settings43. The second NHIS study compared insurance rates between two periods, 2013–2014 and 2017–2018, corroborating the findings of the former study but also noting that rising insurance rates among SM populations appeared to be explained by elevated rates of enrollment in Medicaid44.
These extant studies, however, did not examine differences based on urbanicity of residence or sex, a second gap our analysis fills. We found that urban gay males had higher rates of insurance relative to urban heterosexual males; however, no significant differences were observed for urban bisexual or rural-dwelling SM males. Urban bisexual females, meanwhile, had lower likelihood of having insurance relative to urban heterosexual females while the likelihood of insurance was even lower among rural lesbian females. These data suggest that the previous work observing relative parity in insurance rates between sexual minorities and heterosexuals may have masked variability in disparities based on setting of residence and differences based on sex. Future work should aim to develop a more nuanced examination of these differences in insurance rates based on urbanicity of residence in order to inform future policy decisions regarding expansion of publicly funded programs such as Medicaid under the Affordable Care Act.
Even more robust is a growing literature regarding broad health disparities in chronic disease between sexual minorities and heterosexuals. Past research using data from the National Epidemiologic Survey on Alcohol and Related Conditions observed that not only were sexual minorities at elevated likelihood for a host of chronic diseases compared to heterosexuals but also that bisexual individuals were at increased odds relative to heterosexuals and other sexual minorities45. A more limited sample of young sexual and gender minorities in Chicago noted that not only were sexual and gender minorities at increased risk of cardiovascular diseases, but this likely began at a much earlier age than heterosexuals46 – findings upheld by more recent work using data from the PATH study8. As with insurance rates, however, limited work exists assessing these health disparity differences based on urbanicity of residence.
Our own work here, however, did note several key findings; chief among these is a consistent increased odds for rural bisexual females relative to urban bisexual females across several chronic diseases. Bisexual females as a whole are likely at increased likelihood for physical health conditions as a result of the unique stigma that they experience (ie bisexual-specific stigma, bisexual erasure)17,47-49 and their elevated risk for experiencing sexual harassment and assault compared to heterosexual females5,50. Both bisexual-specific stigma and experiences of sexual assault have been linked to elevated risk for poor physical health17,51. Rural bisexual females appear to experience elevated odds for poor physical health compared to urban bisexual females, while this same pattern of higher odds among rural versus urban sexual minorities is not as prevalent among other SM groups. The reasons for this disproportionate impact of rural residence for bisexual females are unclear; however, potential factors may include higher exposure to minority stress for bisexual females living in rural areas52,53, difficulties maintaining visibility as a bisexual individual in rural settings54, reduced access to high quality and affirming health care and sexual assault services in rural areas55, or reduced access to protective factors for bisexual females in rural communities53.
Four other diseases – diabetes and COPD for rural lesbians, and cancer and kidney disease for rural bisexual females – exhibited lower odds of disease than for rural heterosexual females. These findings, however, have been mixed in past research and their importance here should be tempered as more research is required in this area. For example, one past study has demonstrated that lesbian and bisexual women are at higher risk for developing type 2 diabetes both in general as well as at an earlier age than heterosexual women56, but a more recent meta-analysis across seven studies found no significant increased risk of diabetes mellitus among SM women57. Regarding cancer risk, little research exists in this area among SM women but what does exist notes that they have elevated risk factors, particularly for breast cancer, yet little research has assessed actual differences in risk58.
Although our own findings do contrast those of past research, this may be attributable to low rates of health insurance among SM women, suggesting it is likely these diseases are simply underreported among this population, especially for those dwelling in rural environments. Further, it is important to note that the confidence intervals for these four relatively uncommon diseases in this sample were very wide. Therefore, these estimates may be based on a small number of lesbian or bisexual females with these diseases and, as a result, findings may not be robust or replicable. Future work should aim at improving access to not only basic health care among SM women but quality, SM-informed and focused care. This sort of informed care not only improves outcomes among sexual minorities but also reduces biases among providers themselves27. This has the potential to reduce the burden on the medical system as a whole by increasing sexual minorities’ use of primary care services and potentially reducing downstream health disparities.
Our findings regarding health disparities among sexual minority men were more limited and were largely concentrated among urban populations. Here, gay and bisexual men exhibited elevated risk of a host of diseases including asthma, blood pressure, COPD, hepatitis B or C, and diagnosis of any STI. Interestingly, this likelihood persisted even in light of urban gay men being more likely than urban heterosexual men to currently have some form of health insurance (eg private insurance, Medicaid or Medicare). This may be directly attributable to high rates of internalized homophobia among men who have sex with men, which drives lower engagement in healthcare settings27,28. Thus, even though urban gay men in this sample may be more likely to have health insurance they may also be less likely to seek care and utilize that insurance. These findings have broad clinical and policy implications as they suggest that urban gay men may not seek care in a timely manner, resulting in later diagnosis of health conditions relative to their heterosexual counterparts and potentially increasing burden on healthcare services, each of which can drive up medical care cost. Future research should aim to assess how these factors are coinciding among urban gay men to better understand how to improve health outcomes and whether rural gay men experience similar or different phenomena.
Our study has several limitations that should be considered when interpreting our results. Although NSDUH is a representative survey it relies primarily on self-reported measures bias and may not accurately reflect clinician-diagnosed conditions. NSDUH also excludes incarcerated and homeless individuals from participation in the survey, a study design that may inadvertently reduce the representation of sexual minorities given their higher likelihood of experiencing homelessness59. Minors participating in the NSDUH survey did not receive items related to sexual identity, thus the study does not reflect a significant portion of the SM population. As previously mentioned, sex and gender are conflated in the NSDUH dataset, and as such it is probable that the sample includes gender minorities, but there is no means to examine this. Similarly, options regarding sexual minority status are severely limited and do not allow for the more nuanced analyses that are typically included in studies such as this.
The repeated cross-sectional design of NSDUH limited our ability to assess any potential causal relationships and risk of disease based on residence in an urban or rural environment, particularly as individuals move between these settings.
Lastly, NSDUH is not specifically designed to assess nuanced differences in chronic disease, thus power was limited for anything less than lifetime prevalence, estimates that should be updated using more recent data, such as past-year or past six-month prevalence. Power also limited our ability to examine the rurality variable at the more granular, three-level variable. Estimates of disparities in some less common lifetime physical health conditions produced wide confidence intervals because of small cell sizes. These findings should be considered with caution given that they may not be replicated by future studies with larger samples.
Conclusion
Even considering these limitations, we observed several key findings in this analysis. Key among these is a set of differences in likelihood for physical health conditions by sex and urbanicity of residence, suggesting that rural SM females – particularly rural bisexual females – experience elevated odds for physical health conditions. Secondarily, we observed disparities in insurance status that stand in stark contrast to past research, likely attributable to differences in pooling all sexual minorities into a single risk group versus our stratified approach here. SM females have low likelihood of currently having insurance, with rural-dwelling SM females having low rates. Future research should focus on developing a better understanding of differences based on the setting of residence while simultaneously working with clinicians to provide better informed care of the unique needs of SM populations.
Funding
This work was supported by a grant from the National Institute on Drug Abuse at the National Institutes of Health (R03DA052651, PI: Morgan). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. The sponsor had no involvement in the conduct of the research or the preparation of the article.
Conflicts of interest
The authors declare no conflicts of interest.


