Introduction
Breast cancer currently ranks as the most commonly diagnosed cancer worldwide. According to the most recent global cancer burden statistics, there were an estimated 2.26 million incident cases of breast cancer in 20201. Recent data indicate that breast cancer was responsible for nearly 685,000 deaths among females globally1.
In Brazil, breast cancer stands as the most prevalent tumor type among women, excluding non-melanoma skin cancer, and represents the leading cause of cancer-related mortality. The estimated number of new breast cancer cases in Brazil for the period of 2023 to 2025 is 73,610, corresponding to an estimated risk of 66.54 new cases per 100,000 women2.
Given Brazil's extensive geography, incidence rates vary across regions, with higher rates observed in more developed areas such as the south and south-east, and lower rates in economically disadvantaged regions like the Amazon. For instance, the state of Acre anticipates 100 new cases of breast cancer (with a rate of 26.20 per 100,000 women), of which 70% are expected to be diagnosed in the capital city of Rio Branco2. At the same time, the proportion of breast cancer cases classified as advanced disease (stages III and IV) prior to treatment initiation is notably higher in Brazil's North Region (50.1%), where a shortage of mammography services and specialized healthcare professionals is prevalent3.
The observed survival advantage among breast cancer patients in more developed countries can largely be attributed to a combination of early detection strategies, access to timely diagnosis, and improved access to effective treatments4. Nevertheless, many women encounter access barriers, due either to residing in remote areas distant from healthcare facilities and screening services, or to limited availability of mammography services5. Similar barriers have been identified in underserved rural regions of Sub-Saharan Africa. A qualitative study conducted in Tanzania described multilevel barriers to the early detection of breast cancer, including limited awareness, cultural beliefs, lack of male partner support, poverty, and the absence of preventive health services6. Furthermore, a systematic review of breast cancer screening programs in East Africa emphasized recurring challenges such as inadequate infrastructure, long travel distances, social stigma, and fear associated with a cancer diagnosis7.
Similarly, studies conducted in developed countries like the US and Canada have indicated that women residing in small towns or rural areas, far from cancer diagnostic centers, tend to have lower participation rates in screening programs, consequently impacting survival rates8,9.
Mobile mammography units have been deployed in various countries to mitigate access disparities, particularly among minority populations10. A study in France evaluating the cost-effectiveness of mobile mammography programs identified their potential to increase participation in breast cancer screenings among target demographics and reduce social inequalities, particularly in remote and underserved areas11.
Despite these well-documented challenges, limited empirical evidence is available on the performance of structured breast cancer screening programs in remote regions of the Brazilian Amazon, particularly in the state of Acre. This study aims to address that gap.
The objective of this study was to analyze the implementation of an opportunistic breast cancer screening program in the state of Acre (Western Amazon, Brazil) and assess its initial outcomes.
Methods
In 2019, the Barretos Cancer Hospital, a philanthropic institution headquartered in the municipality of Barretos, state of São Paulo, known for its cancer screening initiatives throughout Brazil, inaugurated a Fixed Cancer Prevention Unit in Rio Branco, the capital of Acre, located in the Western Amazon, named Hospital de Amor Acre. Additionally, two mobile units were deployed to conduct both mammography and pap test screenings in rural areas and the smallest cities around the capital, Rio Branco. This important area was chosen because it lacks an organized breast cancer screening program, and many people in small cities have limited access to specialized care.
The state comprises 22 municipalities, with four accessible exclusively by the river. The population is estimated at 906,876 inhabitants, of whom 52,493 are female and fall within the established age group for biennial mammography screening12. At that time, one fixed unit with an area of 26,910 square feet (approx. 2500 m2) (Fig1) and two mobile units were established, offering services completely free of charge through the Brazilian Universal Health Coverage (Sistema Único de Saúde).
The fixed unit conducts screenings for breast, cervical, skin, and oral cancers. Its multidisciplinary team initially received training at the Prevention Unit of Barretos Cancer Hospital, a reference center for organized breast cancer screening in Brazil, before assuming responsibilities at the Department of Radiology of Hospital de Amor Acre. The unit is equipped to perform diagnostic and complementary examinations (such as localized compression and magnification, ultrasound, preoperative markings, and ultrasound-guided percutaneous biopsies) and initial cancer treatments (nodulectomy and mammary segmentectomy, guided or unguided by wire). Healthcare professionals working in both the mobile units and fixed units receive weekly training via videoconference on quality management and best practices.
The two mobile units were designed for consultations, mammography examinations, and cervical cytology tests (Fig2). Visits are conducted by a multidisciplinary team comprising a nurse, a nursing technician, a radiology technician and a driver. Operating hours are from Monday to Friday, from 8 am to 5 pm. In certain municipalities, examinations are also conducted during evening hours or on Saturdays, depending on local requirements.
Initially, a nurse from our team conducts a preliminary visit to the municipality 60 days in advance to organize activities and train municipal teams regarding screening programs. Public announcements about the mobile unit's visit are disseminated by municipal authorities through radio advertisements, public announcement systems on vehicles, distribution of leaflets, home visits, and posters in healthcare facilities.
The program serves to all municipalities in the state, including the most remote ones (650 km) and those accessible only by river. Mobile units specifically serve the 18 municipalities accessible by land, while women from the four municipalities accessible exclusively by river must travel by boat to a designated point and then take a bus to reach the mobile unit (Fig3).
In cases of suspected neoplasia, women are invited and encouraged to visit the fixed unit for further assessment and diagnostic confirmation. Cases diagnosed with neoplasia at the initial stage (CS 0 and I according to Union for International Cancer Control's (UICC) internationally standardized Tumor, Node, Metastasis (TNM) staging system classification) are treated at the fixed unit itself. However, cases with more advanced staging (CS II, III and IV according to UICC TNM classification) are referred to treatment at the Hospital de Amor Amazônia, a hospital unit within the Barretos Cancer Hospital complex located in Porto Velho, state of Rondônia, or other hospitals in the state of Acre.
To ensure the quality of examinations performed, the units implement rigorous quality control processes, including initial training for nurses, physicians, and radiology technicians; personalized retraining following daily assessment of production quality; equipment quality control through daily, monthly, and semi-annual tests conducted by a physician with international certification; double reading of mammography exams guided by artificial intelligence to facilitate continuous improvement; weekly meetings for protocol adjustment, case discussion, and literature review; and ongoing technical and medical performance monitoring. This quality monitoring has earned the program international certification for breast cancer screening from the Dutch Expert Centre for Screening (LRCB).
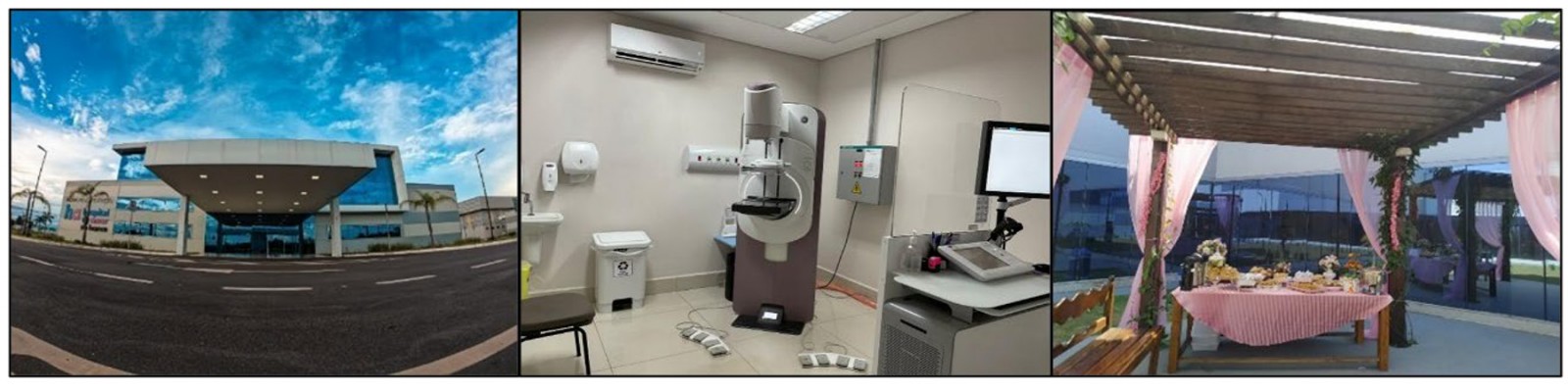 Figure 1: Fixed unit providing mammography services and a welcoming breakfast environment for patients visiting the Rio Branco Cancer Prevention Institute, Western Amazon.
Figure 1: Fixed unit providing mammography services and a welcoming breakfast environment for patients visiting the Rio Branco Cancer Prevention Institute, Western Amazon.
 Figure 2: Mobile unit conducting mammography screenings while reaching out to individuals around Rio Branco with limited access, such as those residing along riverbanks.
Figure 2: Mobile unit conducting mammography screenings while reaching out to individuals around Rio Branco with limited access, such as those residing along riverbanks.
 Figure 3: How the local population around Rio Branco travels to the mobile units for care, from their departure by boat, traveling by bus on unpaved roads, and arriving for consultations.
Figure 3: How the local population around Rio Branco travels to the mobile units for care, from their departure by boat, traveling by bus on unpaved roads, and arriving for consultations.
Data analysis
To evaluate program outcomes, performance indicators from the screening unit were assessed alongside data extracted from the Brazilian Ministry of Health’s cancer information system (SISCAN). The results were compiled and analyzed using Microsoft Excel and described through frequency tables stratified by year.
To assess first-round outcomes of the breast cancer screening initiative, data from 2020 to 2021 were analyzed for women aged 40–69 years who underwent mammography through the Hospital de Amor screening program in the state of Acre, located in the Western Brazilian Amazon. All collected data were organized and tabulated using the Statistical Package for the Social Sciences v22 (IBM Corp; https://www.ibm.com/products/spss-statistics). Results were presented in tabular format, and the χ2 test was used to examine associations between categorical variables.
A quantitative observational design was considered appropriate to evaluate the implementation and early outcomes of a population-based screening program in a real-world setting. This approach enabled systematic collection and analysis of service delivery and diagnostic indicators across a large sample. The χ2 test, a non-parametric method, was selected due to the categorical nature of the variables and the absence of normality assumptions. It is widely used in epidemiological studies to assess proportional differences between groups and was suitable for the descriptive and comparative aims of this initial evaluation.
Although inferential modeling was not applied in this study – due to its exploratory nature and limitations in the available variables – future analyses may incorporate multivariate techniques to investigate predictors of late-stage diagnosis or non-adherence to follow-up protocols.
Ethics approval
This study complies with Brazilian ethical standards and good clinical practice guidelines. Formal approval from a research ethics committee was not required, as the data consisted exclusively of departmental indicators and were fully de-identified.
Although the analysis was based on aggregated and anonymized service data, ethical principles were integral to the program’s implementation. Special attention was given to protecting the privacy and autonomy of women participating in the screening initiative, particularly those from remote and socioeconomically vulnerable communities. Verbal informed consent was obtained from all participants prior to examinations, in accordance with national public health protocols.
Confidentiality was ensured through secure data storage and restricted access procedures. Program personnel received training in ethical conduct and cultural sensitivity to promote respectful and appropriate interactions with participants.
Moreover, the program was intentionally designed to reduce health inequities by engaging local stakeholders and minimizing logistical barriers that might otherwise limit access to care for rural populations.
These measures were essential to uphold ethical standards in contexts where structural vulnerabilities and historical mistrust of the health system may pose additional challenges.
Results
Between 2020 and 2021, a total of 17,680 mammograms were conducted within our cancer screening program in Acre among women aged 40–69 years. The mobile unit commenced its operations in January 2020, conducting 38 visits to municipalities until 2021. Throughout this period (2020–2021), the mobile unit performed 5457 mammographic examinations, accounting for 30.9% of the total examinations performed by the screening program (Table 1). At the time of analysis, 70 mammograms did not have a final report.
The Prevention Institute identified 1705 cases categorized as BI-RADS (Breast Imaging-Reporting and Data System) 0, 4, and 5, out of which 321 (18.8%) were subsequently referred for biopsy (Table 2).
Among these 321 diagnostic procedures, 64 cases (19.9%) of breast cancer were confirmed (29 in 2020 and 35 in 2021). The distribution of breast cancer cases across age groups reveals that individuals aged 40–49 years constituted 28 cases (43.8%), those aged 50–59 years represented 24 cases (37.5%), and participants aged 60–69 years accounted for 12 cases (18.8%). Most of them occurred in younger women (43.8% aged 40–49 years) with 31 cases (48.4%) diagnosed at the initial staging (CS 0, I) (Table 3).
All women diagnosed with breast cancer in this study commenced treatment for the disease within 60 days of diagnosis.
No statistically significant difference was found (p=0.566) in the distribution of clinical staging and age (Table 4).
Table 1: Number of mammograms performed in 2020 and 2021 by the Cancer Prevention Institute, state of Acre, distributed by age group
| Age group (years) |
Fixed unit n (%) |
Mobile unit n (%) |
|---|---|---|
| 40–49 | 6521 (70.3) | 2750 (29.7) |
| 50–59 | 4015 (67.0) | 1975 (33.0) |
| 60–69 | 1687 (69.7) | 732 (30.3) |
| Total | 12,223 (69.1) | 5457 (30.9) |
Table 2: Breast Imaging-Reporting and Data System results for mammograms performed in 2020 and 2021 by the Cancer Prevention Institute, state of Acre, distributed by age group
| Age group (years) |
BI-RADS results, 2020 n (%) |
BI-RADS results, 2021 n (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 4 | 5 | 0 | 1 | 2 | 4 | 5 | |
| 40–49 | 288 (9.77) | 1024 (34.75) | 1579 (53.58) | 25 (0.85) | 6 (0.20) | 599 (9.54) | 1638 (26.08) | 3924 (62.48) | 55 (0.88) | 6 (0.10) |
| 50–59 | 137 (7.28) | 488 (25.93) | 1220 (64.82) | 16 (0.85) | 5 (0.27) | 324 (7.93) | 764 (18.69) | 2889 (70.69) | 48 (1.17) | 18 (0.44) |
| 60–69 | 62 (7.84) | 138 (17.45) | 580 (73.32) | 8 (1.01) | 0 (0.00) | 84 (5.18) | 233 (14.36) | 1267 (78.07) | 19 (1.17) | 5 (0.31) |
| Total | 487 | 1650 | 3379 | 49 | 11 | 1007 | 2635 | 8080 | 122 | 29 |
BI-RADS, Breast Imaging-Reporting and Data System.
Table 3: Distribution of breast cancer cases diagnosed in 2020 and 2021 by the Cancer Prevention Institute, state of Acre, distributed by age group and clinical stage
|
Clinical stage† (UICC TNM) |
Frequency | Percentage |
|---|---|---|
| CS 0 | 7 | 11.1 |
| CS I | 24 | 38.1 |
| CS II | 27 | 42.9 |
| CS III | 4 | 6.3 |
| CS IV | 1 | 1.6 |
† Clinical stage not available for one case.
CS, clinical stage. UICC TNM, Union for International Cancer Control's internationally standardized Tumor, Node, Metastasis.
Table 4: Cross-tabulation of positive cases according to age group and clinical staging of breast cancer diagnosed in 2020 and 2021 by the Cancer Prevention Institute, state of Acre
| Age group (years) | Clinical stage group (UICC TNM) | Total | p-value (χ2) | ||
|---|---|---|---|---|---|
|
CS 0, I |
CS II n (%) |
CS III, IV n (%) |
|||
| 40–49 | 12 (44.4) | 13 (48.1) | 2 (7.4) | 27 | 0.566 |
| 50–59 | 11 (45.8) | 10 (41.7) | 3 (12.5) | 24 | |
| 60–69 | 8 (66.7) | 4 (33.3) | 0 (0.0) | 12 | |
CS, clinical stage. UICC TNM, Union for International Cancer Control’s internationally standardized Tumor, Node, Metastasis.
Discussion
In Brazil, most mammography services are opportunistic and diagnostic-focused. Transitioning to an organized screening model using both mobile and fixed units has been associated with increased adherence and improved outcomes, as observed in similar programs at Barretos Cancer Hospital in São Paulo state13. Successful screening programs commonly rely on community engagement, free access to services, and proximity to healthcare infrastructure14.
Barretos Cancer Hospital has over 30 years of experience in cancer screening, including cervical, skin, colorectal, prostate, and breast cancers, and has expanded efforts to low-income regions with limited healthcare access15-19. Since 2012, the institution has manufactured mobile units tailored to the specific needs and geographic conditions of target areas. These units support screening in remote locations where healthcare infrastructure is limited.
At Hospital de Amor Acre, comprehensive follow-up is provided for all women screened, including prompt recall for abnormalities and coordination of diagnostic and therapeutic services. Women with BI-RADS 1 or 2 results are scheduled for routine rescreening, while those with suspicious findings receive additional evaluation. This integrated follow-up mitigates delays and ensures continuity of care.
A Canadian study has shown that disparities in false positive rates are linked to inadequate quality assurance protocols, including limited reading volume requirements20. Our program emphasizes daily digital quality control and continuous monitoring of professionals to ensure consistency across fixed and mobile units.
The North Region of Brazil, including the state of Acre, is marked by low literacy, income, and human development indexes21. Despite having the highest breast cancer incidence in the region 20, geographic barriers and limited services hinder timely care. In our study, about one-third of mammograms were performed by the mobile unit, demonstrating its critical role in reaching remote areas. Similar results were observed in other Brazilian initiatives22 and international settings such as California23, where mobile screening improves equity and access24.
These findings resonate with global efforts to address rural healthcare gaps. Studies from Canada and Sub-Saharan Africa consistently cite geographic isolation, limited workforce, and logistical challenges as barriers to early detection25,26. In both regions, mobile outreach and referral coordination have emerged as key strategies, reinforcing the relevance of our approach in the Western Amazon.
In Brazil, many states lack structured screening systems. A strength of our program is the consolidation of diagnostic procedures (ultrasound and biopsy) and treatment (early-stage surgery, advanced-stage referrals) within the same system, all provided free of charge. Notably, advanced-stage patients often presented with symptoms and had difficulties accessing public mammography services.
In its first 2 years, the Acre program detected 64 breast cancer cases, with 92.1% at early stages (CS 0–II) and only 7.9% at advanced stages (CS III, IV). According to Instituto Nacional de Câncer data from 2000 to 2015, advanced-stage breast cancer in the North Region accounts for 50.1% of cases3, while early-stage diagnoses are more common in regions with a higher human development index. The early detection rate in this study thus surpasses national and regional benchmarks.
This contrasts with previous findings in Acre, where advanced-stage diagnoses ranged from 39.1% to 57.3%27-29. Studies have linked late diagnoses to individual and contextual factors, including equipment distribution and systemic access barriers27,30. The high prevalence of late-stage diagnoses in Acre has contributed to increased mortality, driven by delayed diagnosis and insufficient treatment infrastructure31.
Although no statistically significant association was found between age group and clinical staging (p=0.566), the predominance of early-stage diagnoses observed in this program suggests its potential for improving early detection among underserved populations.
Human resource limitations also challenge quality control. To address this, our institution ensures strict monitoring of image quality and diagnostic consistency. Moreover, all women diagnosed in this study began treatment within 60 days, in compliance with Brazil’s Law 12732/201232.
The sharp rise in breast cancer mortality in Acre from 1980 to 201433 highlights the urgency of investing in screening and early detection. The Hospital de Amor screening program, with international standards and the use of mobile units, contributes to reducing inequalities and improving access to care in the region.
Beyond addressing the high burden of late-stage breast cancer diagnoses in the North Region of Brazil, the program was specifically designed to overcome structural and logistical barriers to healthcare access, including long travel distances, poor road conditions, and a shortage of specialized medical professionals. To achieve these goals, the intervention integrated mobile mammography units with a fixed high-complexity cancer prevention center, with the aim of expanding screening coverage, promoting early detection, and enabling timely diagnosis.
The program also incorporated quality assurance protocols, continuous training for healthcare personnel, and an integrated referral network to minimize delays in diagnosis and treatment. Together, these strategies were designed to enhance equity in breast cancer outcomes across the Western Amazon.
Nevertheless, limitations remain. Population-level coverage data were unavailable, preventing estimates of reach. Updated mortality statistics for 2020–2021 are also limited, due in part to the COVID-19 pandemic, which affected screening operations. These contextual factors must be considered in interpreting the results.
Another limitation is the inability to separate data by unit type (mobile v fixed) or by indication (screening v diagnostic). As data collection processes mature, such distinctions will enhance future evaluations.
Additionally, qualitative insights from patients and staff were not systematically collected in this phase. Anecdotal reports suggest that trust-building, cultural sensitivity, and logistical support were vital, but structured qualitative methods would enrich understanding. Future studies should explore patient perspectives, barriers, and satisfaction through mixed methods to better tailor services to local needs.
Although the χ2 analysis was suitable for this exploratory assessment, advanced statistical models such as logistic regression or survival analysis were not feasible due to data limitations. As the program evolves, the integration of more robust data and mixed methodologies will allow for more in-depth analysis.
Conclusion
The initial results of the breast cancer screening program in Acre demonstrate high early detection rates and improved access in a historically underserved area. These outcomes are promising but must be interpreted cautiously due to data limitations and the program’s early stage. Continued investment, independent evaluation, and long-term monitoring will be essential to confirm its impact and inform replication in similar settings.
Funding
This article received no specific funding.
Conflicts of interest
The authors declare that there are no conflicts of interest to be disclosed regarding this work.
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
References
You might also be interested in:
2014 - 'Heart attack' symptoms and decision-making: the case of older rural women





