Introduction
For a patient living through cancer, the participation of their general practitioner (GP) in care varies during the disease’s course1,2. The GP has an important role in understanding and exploring symptoms to distinguish cancer from non-cancer3,4 and in palliative care at the end of life5,6. Traditionally, hospital doctors perform the treatment of cancer. However, especially in rural and remote areas, some treatment has been decentralised to GP surgeries and rural hospitals7,8. Many patients want their GP to take an active part in the cancer therapy9. Also, GPs have an important role in psychosocial care and care for side effects of treatment10,11. Supporting patient and family, translating information into lay language and being a mediator between patient and specialist care are also important GP tasks10,12. In areas with long distances to hospital, patients could save travel time if GPs performed more tasks12.
The interface between secondary and primary care has long been recognised as a critical point regarding quality of care13. Communication between lines of health care is often found wanting14. Especially important when sharing care is timeliness of specialists’ communications to GPs10,12. In addition to questions arising during the active phase of cancer treatment, GPs deal with an increasing number of survivors who need assistance based on a profound understanding of cancer sequelae and of possible complications of treatment15. Given their often long-lasting and close relationships with their patients, GPs are in a position to accompany them throughout the whole process of cancer care5,16. However, such longitudinal involvement has been relatively uncommon17.
The aim of this study was to explore how Norwegian GPs participated in different kinds of cancer treatment, and for what forms of cancer. Furthermore, the authors asked what kind of communication the GPs had with patients and hospital doctors, as well as with local health personnel and patients’ relatives. Finally, the authors wanted to discuss what is feasible and appropriate.
Methods
The chief municipal medical officer in 178 Norwegian municipalities in April 2007 received an invitation letter together with enclosed letters to GPs containing self-explanatory four-page questionnaires (Appendix I), modified from a previous study18. The medical chief was asked to identify up to five GPs who might have participated in local treatment of cancer patients in 2006–07 and to forward the questionnaire to them. Thus, the intention was to recruit a rather select group of GPs who had experience from work with cancer patients. All 93 municipalities in North Norway and a 25% random sample (85 municipalities) in South Norway received the invitation. Treatment intention could have been curative or palliative. The anonymous patient could be alive or dead at the time of reporting. In addition to marking pre-defined answers, the GPs were encouraged to give free-text commentaries.
Participating GPs received no remuneration, only a certificate attesting the approximate time they had spent answering the questionnaire. In Norway, such participation in research contributes a limited number of points for the compulsory recertification of GP specialty every fifth year.
Statistics
Chi-square analysis and Mann–Whitney U-test were used to examine differences between groups. Significance level was p<0.05.
Ethics approval
The survey protocol was accepted by the Data Inspectorate of Norway (Project 12962) and ethical approval for the project was given by the Regional Committee for Medical and Health Research Ethics of Northern Norway (P REK Nord 44/2005). Only the individual GP knew the identity of any single patient.
Results
Seventy-eight GPs from 49 (28% of invited) municipalities returned completed questionnaires for 118 patients. The GPs were 36% women and 64% men with an average age of 47 years and an average GP experience of 16 years. Mean age and sex distribution were very close to those of all Norwegian GPs19. There was no difference in participation between north and south. There was a non-significant tendency that GPs in the south had worked a little longer in general practice then GPs in the north – median 20 years and 14.5 years, respectively. Only six of the municipalities were towns with more than 20 000 inhabitants, which means that the majority of patients lived in rural communities or small towns quite far away from hospitals. However, a few localities had rural hospitals14, and most other places had rooms reserved for terminal or other temporary patients in the local nursing home.
The 118 patients comprised 59 women and 59 men. Overall mean and median age was 63 years, median age for women was 61 years and for men 63 years. One patient was a child, and the oldest patient was aged 89 years.
Table 1 shows cancer diagnosis by site, with digestive organs as the major localisation of cancer. All major forms of cancer were represented. Sixty percent of the patients were alive at the time of registration, 16% were in the terminal stage and 12% were in a good condition without ongoing treatment. For 20% of the patients the intention of treatment was curative and the GPs reported that life expectancy at the beginning of treatment was more than 6 months for 63% of the patients. One-third of the patients waited more than 4 weeks before treatment started. The clinical status had been declining for 80% of the patients.
Table 2 shows how the GPs participated in drug and adjuvant treatment of cancer patients. Not counting palliative treatment, 64% of the GPs participated either directly, or indirectly through referrals, in cancer treatment. Seventeen percent of all the GPs participated in administration of chemotherapy, while more than half of the GPs referred patients to other investigations or treatment. The 20 patients who received chemotherapy belonged to no particular diagnostic category, nor were there sex or age differences. Eighty-eight percent of the GPs prescribed some kind of palliative medicine to the patients, especially analgesic and antiemetic drugs. Anxiolytic or antidepressive drugs were prescribed to about half of the patients. Morphine was prescribed equally often from GPs as from hospital. Alternative or complementary medicine was discussed infrequently. Two-thirds of the GPs treated their patients for complications of cancer treatment, with advice from the hospital in 70% of the cases. GPs were involved in the treatment of worsening or relapse for 74% of the patients, most often in cooperation with hospital doctors. For treatment of other disease after cancer was diagnosed, the GPs had contact with the hospital in 41% of the cases. GPs treated patients in several locations, and 59% had some treatment at home. One-third of the GPs reported responsibility for coordinating home care, and two-thirds contributed to different kinds of administrative help.
Table 3 gives an overview of the communication between GP and patient, and of cooperation with the hospital. Almost one-third of the GPs contributed to informing the patient about their diagnosis, and 81% reported having had a thorough conversation with the patient about the patient’s condition and circumstances. Other health professionals and family/friends were important in the care of most patients. In this local setting, a high proportion of the GPs reported that they had had written and/or oral communication with hospital doctors. During the terminal stage most patients were treated at home or at a nursing home; of the patients who died, less than half died at home. The GPs had contact with the family in 72% of the cases after a patient died.
Table 1: Patient characteristics and cancer diagnoses by anatomical site (n=118)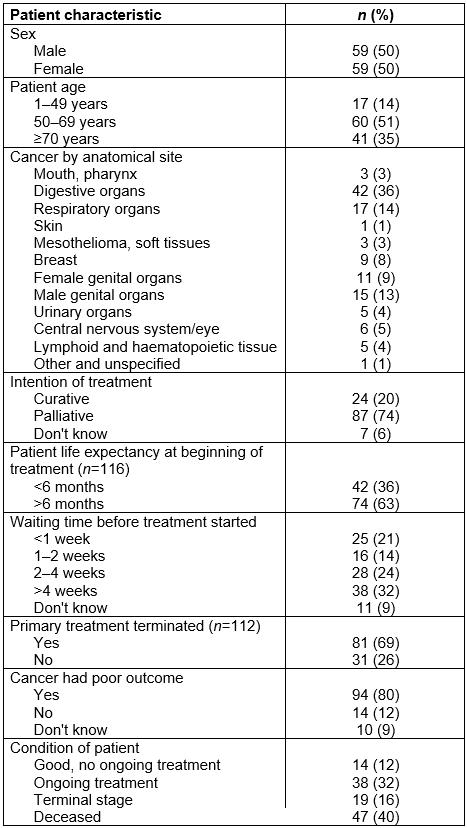
Table 2: General practitioner participation in drug and adjuvant treatment of cancer patients (n=118)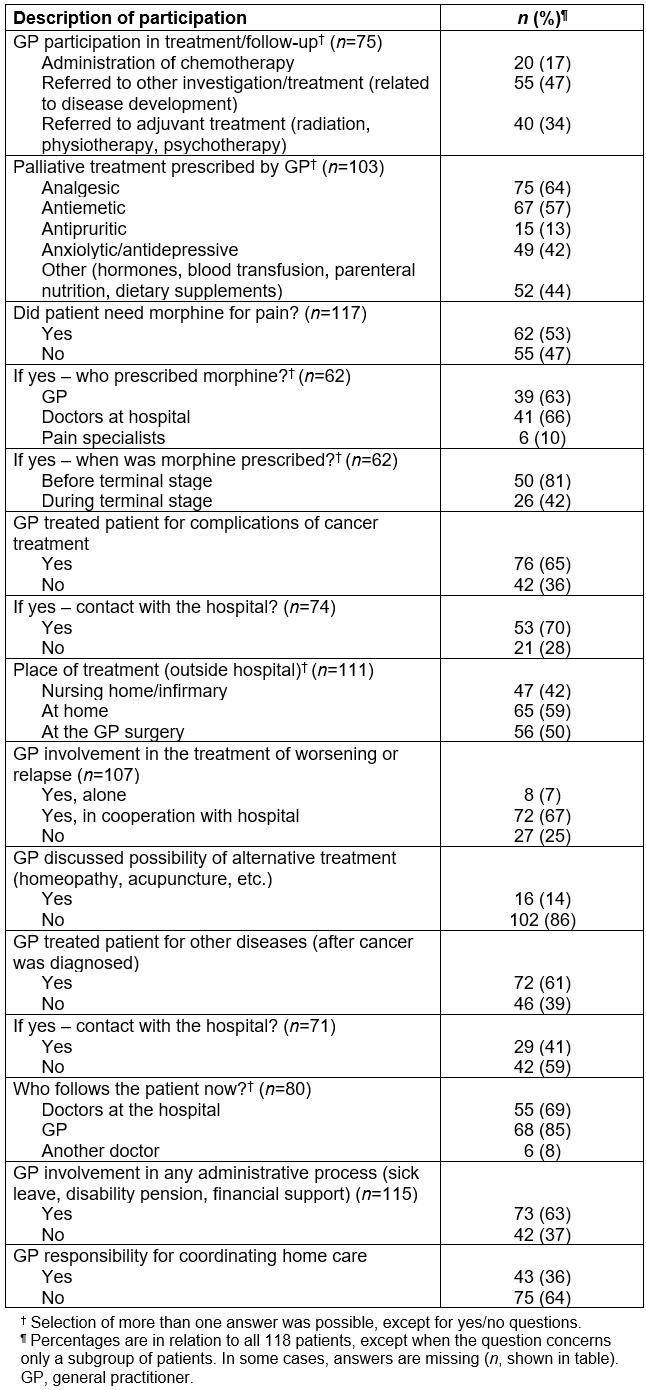
Table 3: Communication between general practitioner and patient, and cooperation with hospital (n=118)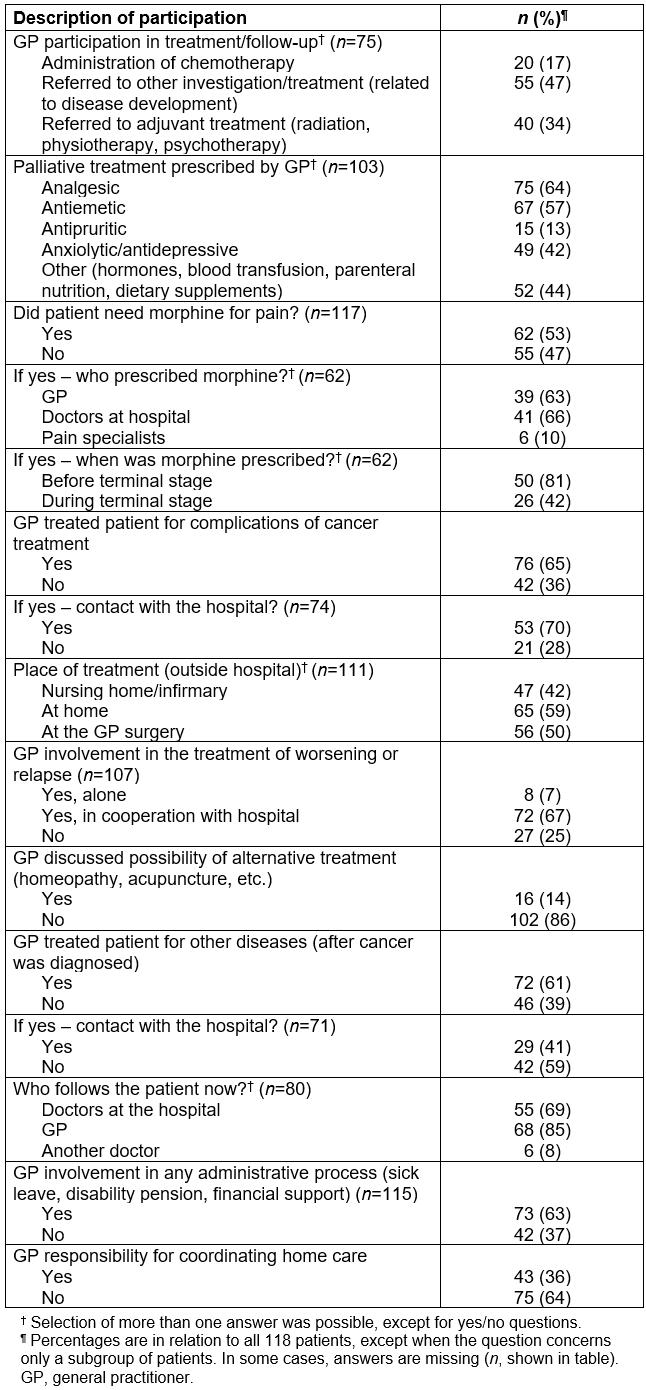
Discussion
Main findings
The study demonstrates that some rural and small-town GPs contributed considerably to cancer care in their patients’ local communities. Most of the patients reported here had progressive disease. Thus, not only the GPs, but also the patients, belonged to a rather select group of cancer patients with a higher than usual need for GPs’ services. For this group, GP participation was substantial not only in palliative care, but also in some aspects of oncological treatment and in clinical follow-up. Most patients received morphine or other analgesic drugs, and the GP had the possibility of both monitoring day-to-day needs and providing prescriptions. The contact with specialist services seemed to be good and useful in most cases. Chemotherapy should and would not be initiated by a Norwegian GP without an initiative and a treatment protocol from an oncologist. Initiation of palliative treatment as well as treatment of complications or disease progression would regularly be discussed with a hospital specialist before the GP acts. After such treatment has been initiated, the GP is more free to adjust dosages.
We may assume that the patient work and the contact with specialists contributed to gradually increased knowledge and skills regarding cancer. Some free-text comments from the GPs supported this. From the GPs’ viewpoint, the communication with the patient also seemed good in this local setting, and there was contact with the family in the majority of cases, both before and after a patient had died. The authors do not have data about the experiences of patients or family carers. However, offering cancer treatment locally is based on an agreement between the patient, the hospital and the local clinicians; the GP and the community nurse, often also an oncology nurse. With local treatment, patients avoid strenuous travelling and might stay in closer contact with their family. The close contact with their local GP and nurses can be a bonus, too.
Less than half of the patients died at home. In recent years, palliative beds in local aged care facilities or in small community hospitals20 have become common places to transfer a terminally ill patient when treatment at home becomes difficult. These palliative units aim for sustained contact between patient and family and continuity of care by local GPs and nurses.
Discussion within the context of the literature
Within the treatment time sequence, GPs may seem to have no role as long as the primary cancer treatment is going on. However, some treatments go on for months, and most patients would appreciate coming home and receiving some of the treatment locally. Pathmanathan et al21 found it safe to administer chemotherapy regimens to rural patients, without increased morbidity or mortality. In rural areas of Australia, GPs have also administered chemotherapy, and the likelihood of this increased with remoteness22. This has also been shown in the northernmost, sparsely populated country of Norway23. In the present study, the GPs reported no problems related to chemotherapy administration in 20 patients living far from hospital services. However, shared care with good communication between primary care and hospitals is necessary in these cases and seems to have been practised. Detailed and timely communication with specialists, particularly about treatment regimens and follow-up care, is often emphasised when GPs are asked how they can contribute to ensure optimal patient outcomes24. A Canadian study25 has shown that women caregivers in rural areas experience considerable challenges in relation to patients with advanced cancer, and that preservation of hope is a crucial factor.
Cancer care may demand quite intensive patient contact over an undetermined time period, and most GPs in one study wanted to limit such a workload to the cases they actually dealt with26. Patients emphasise the GP’s continuity of care and good information that is patient-centred and holistic27. After treatment, their main concern is recurrent disease, and they may doubt that their GP has sufficient expertise to conduct follow-up28,29. Two recent studies from Estonia and France showed that most patients preferred to discuss their cancer-related problems with oncologists, but that patients also contacted their GPs during cancer care, in France often during the initial therapeutic phase of cancer30,31. Geographical questions were not raised in these studies. Altogether, these studies seem to reflect the patients’ well-founded beliefs that a specialist knows more about cancer than a GP, and that a worsening of the condition may be discovered and treated more quickly and appropriately in the hospital. Patients without a trusting relationship with their GPs might doubt their ability to discover and react appropriately to symptoms and signs indicative of cancer. However, for important forms of cancer such as breast cancer and colon cancer, studies have shown that shared care between hospital and GP is acceptable to patients and GPs32-34, and guidelines increasingly recommend delegation of specified follow-up elements to GPs. Close contact with a GP during and after cancer treatment could give good possibilities of discussing personal preferences and therapeutic options.
After the primary treatment has been terminated, studies have suggested that, for most patients, GPs temporarily are less important. Two randomised studies found no effect on quality of life of increased involvement of the GP during this phase35,36. It is possible that many patients need to feel they are regaining health and want to minimise contact with health services beyond regular controls, most often offered in hospital. This does not mean that the GP is superfluous at this time. Patients may still need the GP for other ailments. For cancer, shared care already from the time of initial treatment is appreciated by patients36 and becomes important with time14. Often, survivorship poses new medical problems where the GP becomes increasingly important37,38, and GPs can contribute to detection of recurrences39. To some extent, re-establishment of the contact between patient and GP can be helped by a proactive attitude of the GP40. A model for pro-active cancer care in primary care has been tested in the UK, helping to structure consultations and cover psychosocial areas41. The present study seems to testify that the need for contact with a GP usually increases if the cancer progresses and the patient approaches end-of-life care. The GP then gets important challenges concerning skills, patient–doctor communication and collaboration with other health personnel42.
Strength and limitations of findings
It may be considered as a major limitation that several years have passed since the answers were collected. The article is the last in a series of four articles in a study entitled ‘Optimization of cancer diagnosis and treatment in family practice’, and it has taken time to analyse and write the articles. Previous articles have dealt mainly with the diagnosis part39,43,44. However, geographical conditions have not changed in Norway, and the authors do not think the practice described has changed much since data was collected. It may be seen as an advantage that findings can now be discussed on the background of several recent publications.
Both patients and GPs are special in this study. Patients generally belonged to the unfortunate group with progressive disease, and most of them lived in rural communities or small towns. They belong to a group of patients who have a greater than usual need for GP services. This article presents ideas about how to deal with such patients, allowing them to remain as much as possible in their home and local environment. Only a minority of GPs in each municipality were expected to have been active in recent local cancer care. The local chief medical officer was therefore asked to help select GPs who had been involved in such care, and to send them a request to participate in this study. This may be considered a strength of the study if it means that these GPs were devoted to their tasks and thus gave examples of dedicated care. It is not known to what extent these GPs had previous experience with cancer treatment. However, because most of them worked in remote areas with access to rural hospital beds, this is probable for many of them. Most doctors also had long experience as GPs. Because this study aimed for answers from GPs who had practical experience with cancer treatment, the low response rate is less important.
The information was collected retrospectively in a questionnaire, mainly with pre-selected categories where recall may be imperfect and answers may not fit in the categories offered. However, cancer patients in poor condition are not frequent in a GP practice and are often well remembered by the GP. GPs probably also consulted their medical record notes when completing the questionnaires, as suggested in the letter to the GPs in Appendix I. Free-text comments did not suggest difficulties in reporting. Altogether, the findings gave a coherent picture of dedicated GPs working with very ill patients staying close to their home. In retrospect, it would have been interesting to know more about how the GPs cooperated locally with other health professionals, such as cancer nurses and community nurses.
Conclusion
This study contributes a description of many types of cancer care performed in general practice, in patients with quite advanced disease. Through this, it also contributes an increased understanding of what is feasible and appropriate GP work for such patients. In this select group of GPs, participation rates were high for most of the therapeutic and communicative tasks suggested in the questionnaire, and there was an obvious need for such services. In spite of some understandable skepticism on the part of patients, studies have shown that a close contact with a local GP during and after cancer treatment may be beneficial from many points of view. The optimal way to organise cancer care may vary in different kinds of communities, and more research about this, and about the GP’s role, is pertinent. The closeness of the GP to the patient probably was important when a patient’s clinical condition changed. Challenges may be most complex but at the same time most interesting in communities far from hospitals.
Acknowledgement
Thanks to the 78 GPs who collected data for one or more of their patients where they had contributed to cancer care in the local setting, and to the chief municipal medical officers who tried to identify and recruit GPs who had actively participated in cancer care. Thanks to Tommy Thorsen for help with data collecting, and to Hege Skogstad Berntsen and Per Baadnes for technical assistance with forms and data filing.




