Introduction
Subcutaneous fungal masses resulting from traumatic inoculation of the skin can arise in both immunocompromised and immunocompetent patients1-3. Although traditionally thought to be a disease of the tropics3, it has a global distribution, with an increasing number of fungi now found to be pathogens1-3. An emerging group of fungal infections associated with the development of subcutaneous masses is the dematiaceous (dark brown–black pigmented) moulds. These fungi reproduce asexually and rarely by spore. They are saprophytic and feed on decaying plant material1,4,5.
The genus Rhytidhysteron is a group of dematiaceous pigmented moulds infecting plants and considered to cause fungal disease in humans. Most human infections are reported from India, with two different types of localised subcutaneous fungal masses, known as chromoblastomycosis6 and phaeohyphomycosis, described7. These infections occur after local inoculation of contaminated soil7. Subcutaneous masses due to Rhytidhysteron species have been reported in both immunocompetent and immunosuppressed patients7,8. Rhytidhysteron is thought to have a tropical worldwide distribution in nature7.
This case is presented, to the best of the author’s knowledge, as the first published case from Australia of Rhytidhysteron causing invasive phaeohyphomycosis. It highlights the need to consider fungal causes in the differential diagnosis of subcutaneous masses. This needs to occur globally, rather than being limited to the tropics9. This case is important as the patient’s history suggests that infection has resulted from contaminated soil and plant material in a temperate area of Australia.
Additionally, this case provides empirical observations of the treatment of Rhytidhysteron infection. The patient’s treatment deviated markedly from present recommendations for prolonged antifungal therapy for Rhytidhysteron infection. This was needed as the patient had marked liver disease due to hepatic graft-versus-host disease and blood-transfusion-related iron overload. A deterioration in his liver function reduced his tolerance of the recommended antifungal therapy. Despite this variation in treatment guidelines, he had achieved remission of his Rhytidhysteron infection for 3 years at the time of writing with a combination of surgical and brief antifungal therapy.
Informed consent to publish this case history was obtained from the patient.
Case report
History
A 53-year-old farmer presented to his general practitioner in 2016 with a 3-month-history of an enlarging painless subcutaneous cystic nodule on the medial aspect of his left knee. Due to the position, a pes anserina bursa was queried initially.
He had a complex past history of acute biphenotypic leukaemia, diagnosed in 2014 and treated by sibling allogenic stem cell transplant. The transplant was complicated by chronic hepatic graft-versus-host disease. His past history also included transfusion-related iron overload treated by recurrent venesections.
At the time of presentation of the knee mass, his leukaemia was in remission. He had chronic hepatitis attributable to his chronic hepatic graft-versus-host disease and iron overload. He was treated with tacrolimus and prednisolone to prevent rejection of his allogenic stem cell graft and to reduce progression of the graft-versus-host disease. He received atovaquone to prevent pneumocystis, and valaciclovir to prevent herpes zoster. He received human normal immunoglobulin infusions to passively boost immunity. He had regular therapeutic venesections for iron overload.
From 1979 to 1983, the farmer worked with cattle and horses in the tropical parts of the Northern Territory. There was no other travel history and no knee injury at this time. He worked as a farmer in north-western New South Wales from 1984 onwards. He recalled a knee injury in 2004, where he deeply grazed his left knee on a farm implement, with the wound contaminated by soil and wheat dust. It healed with apparent full recovery. There was no recent history of knee trauma. At the time of presentation, he denied weight loss, night sweats and was otherwise asymptomatic. He had noticed no other masses or skin rashes.
Examination
On examination, the patient was afebrile, with normal observations. He was in no obvious distress. The mass was 2 cm in diameter, situated at the medial aspect of his left knee. It was mobile with no fixation of skin or deeper structures. It did not fluctuate or transilluminate. There was full range of movement in the knee. His ligaments were intact. There was no dermatological abnormality in the skin, hair or nails to suggest fungal infection. There was no palpable lymphadenopathy or hepatosplenomegaly.
Investigations
The patient’s full blood count did not suggest leukaemia recurrence, showing borderline lymphopenia at 0.9 × 109/L (normal 1.0–4.0 × 109/L). His electrolyte profile showed renal impairment, with creatinine 128 µmol/L (normal 60–110 µmol/L) and urea 11.6 mmol/L (normal 3.1–8.1 mmol/L). There was hepatic impairment, with elevation of most liver function tests, notably a gamma-glutamyl transferase of 796 U/L (normal 12–64 U/L). His ferritin was elevated, at 3605 µg/L (normal 30–300 µg/L), consistent with secondary haemochromatosis resulting from recurrent blood transfusions.
The mass was excised under local anaesthesia as ultrasound showed heterogenous echos and shadows inconsistent with a simple cyst or lipoma. The histology showed normal skin with subcutaneous granulomatous inflammation and suppurative granuloma with cystic change centrally. This was surrounded by epithelioid cells and multinucleated giant cells. No foreign body or crystals were seen on polarised light. Pigmented fungal spores and hyphae were identified within the granuloma. There were no fungal bodies seen.
A wound swab of abscess fluid failed to show fungal elements or acid-fast bacilli on microscopy. A dark mould grew after 2 weeks, which was referred to the Royal North Shore Hospital reference laboratory in Sydney for identification. This sample was subcultured onto Sabouraud’s agar with antibiotics and potato dextrose agar, and incubated at 37ºC and 28ºC. The colony was initially pale grey in colour, floccose in texture and with age became black. Reverse appeared dark in colour. Optimal growth seemed to be at 28ºC. There appeared to be no growth on agar containing cycloheximide. Microscopy showed septate, smooth-walled, melanised hyphae with no sporulation.
After 6 weeks of incubation with still no sporulation the isolate was referred on for molecular 16S rRNA internal transcribed spacer fungal polymerase chain reaction (ITS fungal PCR) sequencing (conducted by the molecular laboratory of the Royal North Shore Hospital; technical details of methods and reagents are available from the author). This was identified as a 99% match to Rhytidhysteron species. Species identification was not possible as the specimen did not produce spores. A CT scan of the brain, chest and abdomen did not show any other fungal masses.
Treatment and progress
The patient commenced on an antifungal, posaconazole. About 2 weeks later, he became unwell with lethargy, right upper quadrant pain and vomiting. An abdominal ultrasound showed fatty liver with absence of cholelithiasis or cholecystitis. His liver functions had worsened with gamma-glutamyl transferase of 995 U/L. However, his alanine transaminase was 164 U/L and aspartate transaminase was 74 U/L, remaining fairly stable. It was unclear whether this change reflected graft-versus-host disease or was a drug effect of posaconazole. It was elected to cease the posaconazole until liver functions improved and to observe the knee wound for local recurrence.
Six months after initial presentation, a 4 mm mass was palpable in the left knee scar. This increased in size over a fortnight, and a recommendation to re-excise the scar and mass was made. Figure 1 shows the surgical scar with normal skin and recurrent mass at this time. The mass had a rapid growth, increasing to a diameter of 2 cm in 2 weeks. Histology showed granulomas with spores and hyphae. A fungal culture grew Rhytidhysteron species, identified by 16S rRNA ITS fungal PCR sequencing. Posaconazole was recommended, but the severity of the patient’s liver disease prevented this from being recommenced.
Fourteen months after initial presentation, the left knee mass recurred. A decision for repeat wide excision under general anaesthesia was made. The mass was 3 cm × 2 cm, and 1.5 cm thick, located just under the skin. It had stained surrounding fat a grey colour. This most likely arose from the fungal pigments produced by the recurrent Rhytidhysteron infection. The scar was re-excised and extended inferiorly using diathermy to excise and hopefully sterilise the wound. While not optimal, 3 mm skin margins were possible to allow for primary closure. The mass and stained fat were dissected with diathermy. The wound was then lavaged with iodine solution, waiting 4 minutes for it to take effect. The wound was then washed with 150 mL of 3% hydrogen peroxide. Normal saline was then used to wash the wound. The skin was closed with prolene sutures. No subcuticular sutures were used. No local anaesthetic was used. By using these methods, the potential for spreading spores further was reduced10. Repeat histology directly after surgery revealed granulomatous inflammation. Fungal elements on this occasion required periodic acid–Schiff stain for fungal elements to be seen. These fungal elements extended to the excision margins.
In view of the recurrent nature of the Rhytidohysteron infection and extension of fungal elements to the excision margins, posaconazole was again initiated. This was tolerated for a month. It was ceased when the patient again developed worsening liver function tests with abdominal pain. A decision to cease antifungals was made as the risks to the patient’s liver were considered greater than the risk of a localised phaeohyphomycosis recurrence at his left knee.
The patient’s liver function tests remained elevated for a further 4 months after ceasing of the posaconazole and his presentation was felt in retrospect to be more likely due to worsening graft-versus-host disease. Voriconazole was recommended as an alternative antifungal for this patient. It could not be initiated due to his worsening liver disease.
Surveillance biopsies of scar, skin and subcutaneous fat did not detect Rhytidhysteron infection at 4 months after final surgery. Three years after the most recent excision the patient showed no signs of local recurrence. His liver had improved and his ferritin had reduced with regular venesection. Tacrolimus therapy had reduced his graft-versus-host disease. His gamma-glutamyl transferase at last blood test was 150 U/L (normal 12–64 U/L).
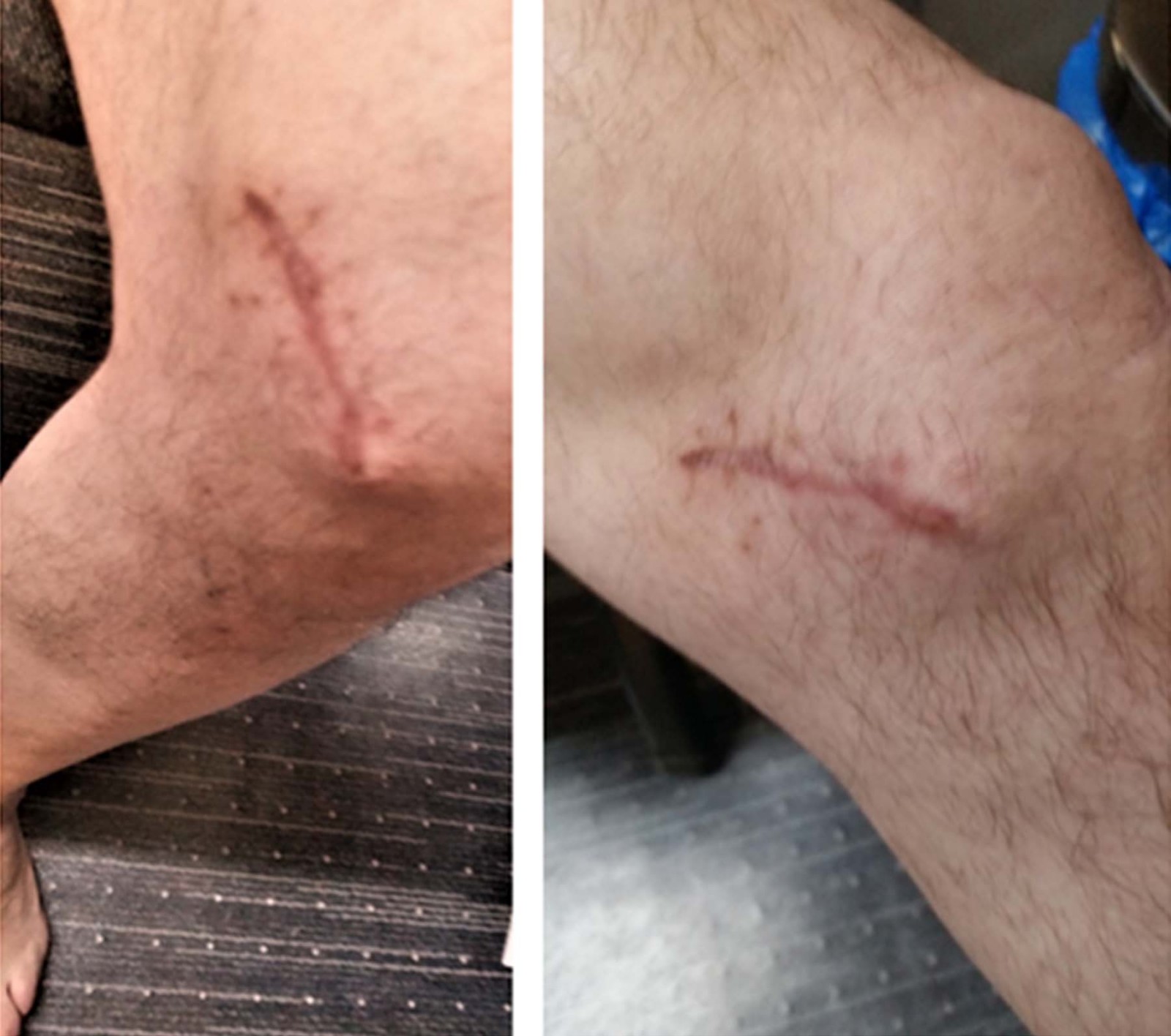 Figure 1: Two views of second recurrence of subcutaneous phaeohyphomycosis mass arising in the knee scar; skin is showing recent scar. It is otherwise normal. (The initial mass had a similar appearance. It was not photographed as it resembled a subcutaneous cyst or lipoma.)
Figure 1: Two views of second recurrence of subcutaneous phaeohyphomycosis mass arising in the knee scar; skin is showing recent scar. It is otherwise normal. (The initial mass had a similar appearance. It was not photographed as it resembled a subcutaneous cyst or lipoma.)
Discussion
Factors in increasing prevalence of fungal infections
This case is presented to highlight that invasive fungal infections are becoming increasingly common in clinical practice1. Several factors are responsible for this. Patients in are now more likely to survive carcinomas, autoimmune diseases and HIV, resulting in an increased number of patients living with long-term immunosuppression. Additionally, diagnostic techniques such as PCR, ITS sequencing and mass spectrometry have improved the identification of difficult-to-culture fungi1,4,5.
The patient in this case report most likely contracted his initial Rhytidhysteron infection from contaminated soil in north-western New South Wales. The fungus reactivated 12 years after inoculation. This coincided with immunosuppression associated with his stem cell transplant for leukaemia. This case study suggests Rhytidhysteron phaeohyphomycosis can develop in temperate Australia
Clinical patterns of subcutaneous fungal disease
Three clinical patterns of disease can arise after subcutaneous inoculation of fungal material. These can be classified as mycetoma (more specifically eumycetoma), chromoblastomycosis and phaeohyphomycosis. Their classification is based on clinical and pathological findings, with some overlap between these groups. Based on this classification, this case study represents subcutaneous phaeohypomycosis due to Rhytidhysteron infection. This is because hyphae are present without grains and sinuses (associated with eumycetoma) or muriform bodies (associated with chromoblastomycosis)4,11.
Risk of recurrence
The surgical scar of this patient needs to be monitored for recurrence. In this case study, pigmented fat was used to define the extent of Rhytidhysteron infection and this was excised. This proved ineffective to define the extent of disease as microscopic spores extended to the surgical margins.
Surveillance biopsies of the surgical scar skin extending to the subcutaneous fat were reassuring, as they did not show overt fungal infection at 4 months. However, biopsies do not sample the entire surgical site and fungal spores may remain dormant in the unsampled sites.
Recurrence will not necessarily coincide with worsening immunosuppression in this patient, It may occur while this patient’s immunity is relatively improved. Patients with normal immunity have developed subcutaneous phaeohyphomycosis due to Rhytidhysteron infection previously8.
If phaeohyphomycosis did recur, the patient’s liver function had further improved at the time of writing and he would be more likely to tolerate voriconazole or other antifungals. Local infiltration of amphotericin B has been used in patients previously to treat localised Rhytidhysteron subcutaneous phaeohyphomycosis12. The interaction between the patient’s immunosuppressants and this drug would need to explored before using this therapy.
Conclusions
This case supports evidence that Rhytidhysteron species occurs in temperate regions of Australia and can cause invasive subcutaneous fungal masses. It is likely to be an emerging global pathogen, more than a tropical disease. In the patient in this case report, optimal treatment needed to be modified because of intercurrent hepatic disease. It is reassuring that there has been no recurrence after 3 years with the approach of general anaesthesia and wide excision using diathermy and lavage of the wound combined with a 1-month course of posaconazole. Further research is needed into how best to treat this emerging pathogen. Fungal infection is becoming more common in practice and needs to be considered in the differential diagnosis of a subcutaneous masses in all patients globally.
Acknowledgements
I wish to acknowledge the contributions of Kerry Weeks and Leonie Chan, NSW Health Pathology, Microbiology Department, Mycology Laboratory and Microbiology Laboratory (respectively), Royal North Shore Hospital, Sydney, who provided mycological advice in revisions of this article.
References
You might also be interested in:
2021 - Tuberculosis in the Torres Strait: the lady doth test too much
2020 - Strategies to increase the pharmacist workforce in rural and remote Australia: a scoping review
