full article:
Introduction
Pivotal to the control of tuberculosis (TB) is the ability for TB control programs to rapidly diagnose and treat the most infectious pulmonary TB (PTB) cases1. Until 2007, WHO recommended three sputum specimens should be collected for the diagnosis of PTB2. However, a large scale sputum microscopy analysis conducted in 42 high volume laboratories showed that the third specimen had a minimal contribution to case detection rates, with an incremental yield of only 0.7% to 7.2%3. A systematic review of 37 studies confirmed the high diagnostic yield for the first two specimens with a minor increase in sensitivity of 2–5% with collection of a third specimen4. Currently, WHO recommends a minimum of two sputum samples, which has the benefit of reduced time to diagnosis and treatment initiation with improved retention of patients in the care pathway2.
In the Torres Strait Islands, Australia, standard practice still involves the collection of three early morning sputum samples or three samples over three consecutive days or three samples over 2 days using the ‘spot-morning-spot’ protocol, for local residents5. In Papua New Guinea (PNG), two samples are required, following the ‘early morning-spot’ protocol6.
The Torres Strait Islands sit adjacent to the Western Province of PNG where TB is endemic and previous studies have demonstrated cross-border transmission of drug-susceptible and multidrug-resistant (MDR) TB7. TB diagnostic services (including specimen collection) have long been provided to PNG nationals permitted to travel to 14 Torres Strait communities in the Torres Strait Protected Zone (TSPZ) without passport or visa8. Primary health centres (PHCs) in the TSPZ are minimally staffed, with many one-nurse posts, and only two PHCs have X-ray facilities. TB diagnostic technology is not available in the Torres Strait and the distance from the northernmost Australian island in the TSPZ and the Queensland Mycobacterium Reference Laboratory in Brisbane, where testing occurs, is 2318 km away.
Aside from culture confirmation, TB is diagnosed using smear microscopy to detect acid-fast bacilli (AFB) in sputum by using DNA detection technology such as Xpert Ultra®, which can detect the presence of Mycobacterium tuberculosis and likely rifampicin resistance within 2 hours9. Rifampicin and isoniazid resistance constitute MDR-TB9. Xpert Ultra has greatly increased sensitivity compared to conventional sputum smear microscopy and its use in a decentralised fashion is now recognised as the preferred frontline test, especially in settings with high rates of MDR-TB10,11.
The turnaround time for conventional microscopy from samples collected in the Torres Strait Islands ranges from a few days to a week and for culture is approximately 6 weeks. In remote settings, difficult cold storage and delayed transport lead to high rates of bacterial overgrowth, which may further delay reporting of results12.
According to the Pathology Queensland laboratory information system, AUSLAB, a poor quality specimen (low volume, salivary contamination) for the diagnosis of M. tuberculosis is ‘that which is less than what is required bacteriologically for diagnosis’. In Queensland, macroscopic evaluation of sputum is undertaken by a laboratory technician to determine good or poor quality and quantity of specimens for testing; however, few studies have assessed the effect of sputum quality on the detection of M. tuberculosis13. Poor quality also refers to samples that are overgrown with contaminating organisms due to poor storage or dispatch delays, particularly in tropical climates14. Furthermore, adequate collection, documentation, packaging and transportation are arranged by clinicians and attention to detail is required to correctly match patients to specimens as well as ensure appropriate collection modalities are used14.
The first aim of this study was to determine the diagnostic yield of sputum specimens collected in the remote Torres Strait Islands from Australian residents and PNG visitors. Collection modality, labelling and transportation of specimens in remote tropical locations were also examined for their effect on diagnostic yield. The second aim was to explore how improved decentralised diagnostic capability may assist earlier diagnosis and treatment initiation in remote settings. This international border, which allows free movement for designated traditional inhabitants without passport or visa, is a biosecurity risk15. Accessing health care is not a provision of the border agreement15 and limited healthcare options for PNG visitors may lead to patients presenting late in their disease course, with advanced signs and symptoms of TB. Both a reduction in the number of sputum specimens required, and more rapid and decentralised diagnoses, have the potential to shorten the time to treatment initiation and increase retention of patients within the care pathway.
Methods
This is a retrospective study of all Australian residents who presented to a health facility in the Torres Strait Islands, and all PNG visitors who presented to a health facility within the TSPZ with clinical signs of TB, between 1 January 2000 and 31 December 2018. Patients were included if at least three specimens were collected within 2 weeks of each other, prior to commencement of treatment; and at least one specimen was positive for M. tuberculosis on culture or nucleic acid testing. Patients who had required medical evacuation to an Australian hospital outside of the Torres Strait Islands were excluded because collection modalities used in tertiary facilities are more advanced than those available in the Torres Strait Islands. A case was defined as smear-positive if one or more sputum samples tested positive for AFBs within 60 days of a positive Xpert M. tuberculosis/rifampicin (MTB/RIF) (Cepheid; https://www.cepheid.com/en/tests/Critical-Infectious-Diseases/Xpert-MTB-RIF) or M. tuberculosis culture. A case was defined as smear-negative if all three sputum samples were defined as negative for AFBs within 60 days of a culture positive result.
Data collection and management
A list of residents of the Torres Strait Islands and PNG visitors diagnosed with PTB within the Torres Strait and TSPZ respectively between 2000 and 2018 was obtained from Queensland Health’s Notifiable Conditions System. All sputum smear results were extracted from AUSLAB and individually reviewed. Four cases were removed from the dataset as laboratory confirmation by Xpert or culture was not represented in AUSLAB. AUSLAB was also manually searched to retrieve data that had the potential to affect laboratory analysis and adversely affect diagnostic yield including sample quality, quantity and collection modality, as well as labelling and packaging errors.
A clinical audit was undertaken to obtain demographic data and chest X-ray results from the electronic patient database used in the Torres Strait Islands, Best Practice as well as state-wide patient information systems HBCIS, The Viewer, Enterprise PACS and XERO. Chest X-ray images without reports or notes by a TB physician directly involved in the patient’s care within patient charts were reviewed by a Queensland Health TB physician to identify lung cavities.
Samples collected for AFB microscopy, culture and phenotypic drug susceptibility testing were routinely tested at the Queensland Mycobacterium Reference Laboratory in Brisbane, a WHO-designated Supranational Reference Laboratory16. Since November 2010, the Xpert MTB/RIF assay has been used on all new sputum smear-positive samples and, upon request, for smear-negative and extrapulmonary specimens16.
Peripheral blood specimens were collected for HIV serology testing when possible.
Data analysis
Frequencies and percentages were calculated for age, age group, gender, country of birth, visa status and HIV serology. Descriptive statistics were generated using the Statistical Package for the Social Sciences v25 (IBM; http://www.spss.com). Diagnostic yield calculations were performed in Microsoft Excel. Incremental yield was calculated for 143 PTB cases diagnosed between 2000 and 2018. All other data were analysed using SPSS. P-values less than 0.05 were considered statistically significant for all tests.
Ethics approval
Ethics approval was granted in writing by the Far North Queensland Human Research Ethics Committee (HREC) (HREC/17/QCH/74-1157) and the Chair of James Cook University HREC, (H7380).
Results
Table 1 provides a summary of the characteristics of all laboratory-confirmed PTB cases diagnosed in the Torres Strait between 2000 and 2018. The majority of the patients from whom at least three sputum samples were collected were adults aged 15–59 years (106/143; 74%) and residents of PNG (114/143; 80%) with an equal gender distribution (female 73/143; 51%). Of all patients diagnosed with PTB, 60% (150/248) were PNG Treaty visitors. Nearly all Australian residents who had a diagnosis of PTB met criteria for the study, with only three excluded for not having had three sputum specimens collected. Overall, 4/248 patients diagnosed with laboratory-confirmed PTB tested positive for HIV, of whom two were included in the analysis. The remaining two HIV-positive patients were excluded as they were not diagnosed with culture-positive TB.
Table 2 shows the cumulative diagnostic yield for patients with at least three sputum specimens collected. Of these 143 patients, 42 (29%) had smear-negative PTB and 101 (71%) had at least one AFB-positive smear. Of the cases that tested AFB positive on any smear, 79% of cases yielded a positive result on the first sample and 96% of cases were positive within the first two samples. Of the 353 good quality samples that tested AFB positive on any smear, 96% of specimens yielded a positive result within the first two samples collected. Among children aged less than 15 years who tested AFB-positive on any smear, 93% of cases were positive within the first two samples. This proportion was similar regardless of quality of specimens. Overall, among the whole cohort, of the 45 poor quality specimens that tested AFB positive on any smear, 96% of specimens yielded a positive result within the first two samples collected. Of the 42 smear-negative patients, five patients had three poor quality specimens.
Table 3 provides a summary of specimen collection modality and age, and how these factors are associated with AFB smear status. The most common route of collection for the samples assessed in this study was voluntary expectoration (391; 91.14%), and 231 (59.08%) of these were AFB-positive PTB cases. The next most common collection modality was nasogastric aspiration (26; 6.06%), followed by nasopharyngeal aspirate (8; 1.86%) and induced sputum (4; 0.93%). Two of the six nasopharyngeal aspirates collected from children aged <5 years, and 2 of the 14 nasogastric aspirates collected from children aged <5 years were AFB-positive. Of children aged <5 years who voluntarily expectorated, smear-positive results were achieved from 75% of the samples collected, and of children aged 5–9 years, 33% of the samples collected were smear-positive.
Multivariate analyses of factors associated with AFB smear-positive results were performed. For all sputum specimens collected (n=429), quality samples were 3.350 times as likely to yield a smear-positive AFB result, and voluntarily expectorated samples were 5.894 times as likely to yield a smear-positive AFB result (p<0.001). Of the 429 samples collected, 76 (18%) were poor quality and, of those, 67% (n=51) were smear-negative.
Table 4 shows univariate and multivariate analyses associated with a smear-positive AFB result. Adults aged 15–59 years, PNG nationals and cavitary disease were associated with a higher odds of any smear-positive AFB result. The regression coefficient for a smear-positive result in adults aged 15–59 years was significant (p<0.05). Univariate analysis demonstrated that patients with smear-positive PTB across all age groups in this study were significantly (odds ratio 2.91; 95% confidence interval 1.12–7.60) more likely to have cavitary disease. Variables assessed in univariate analysis that had a p-value greater than 0.05 included gender, visa status, Treaty status and other age groups, and were not included in the multivariate analysis.
Clinician/clinic-based variables (registration, labelling, packaging errors) did not affect the diagnosis of PTB. Of the 429 individual samples included in the study, samples of 15 patients were affected as 44 were labelled incorrectly. Of those, 41 samples did not have collection time recorded. For the remaining three specimens with a labelling error, the specimens were not tested as the name of the patient on the pathology request form did not match the name of the patient on the specimen container. Among four samples not tested, three samples were considered too old and another leaked in transit.
Table 1: Characteristic of all patients diagnosed with pulmonary tuberculosis in the Torres Strait Islands between 2000 and 2018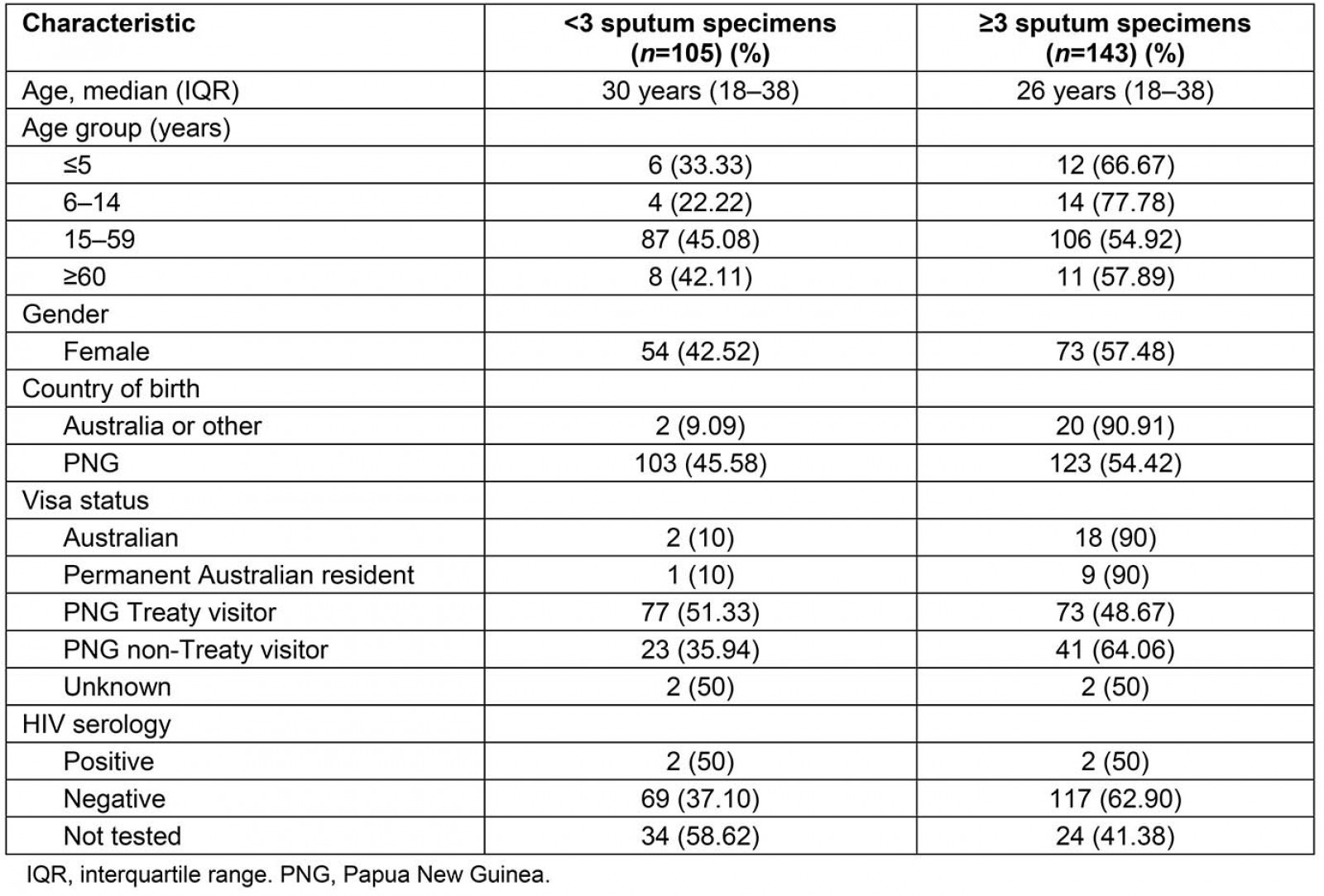
Table 2: Cumulative diagnostic yield from consecutive sputum specimens 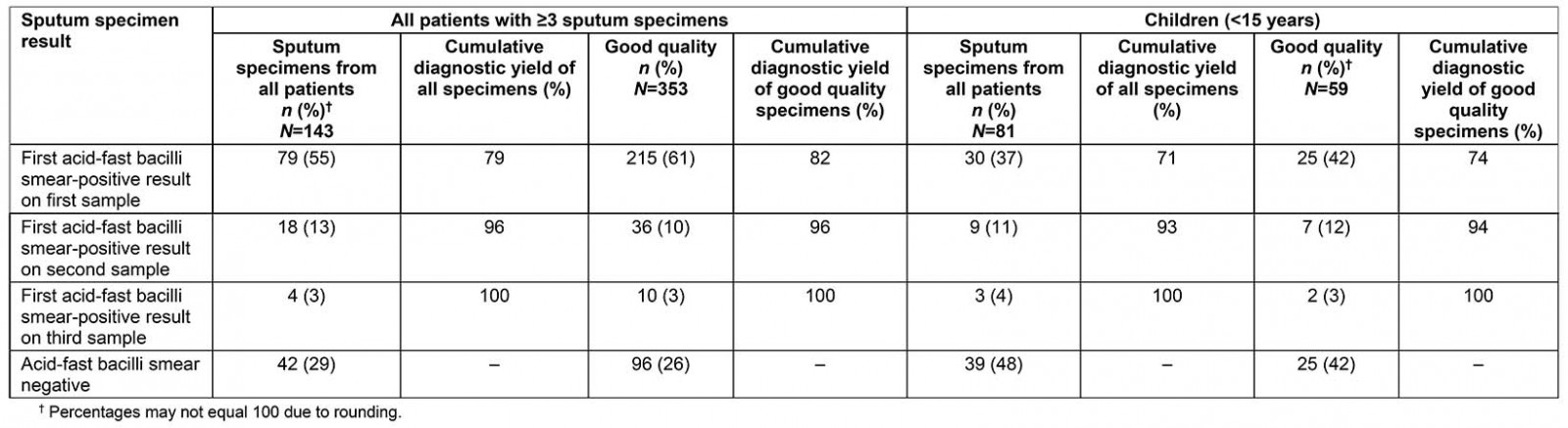
Table 3: Specimen collection modality, age and acid-fast bacilli smear status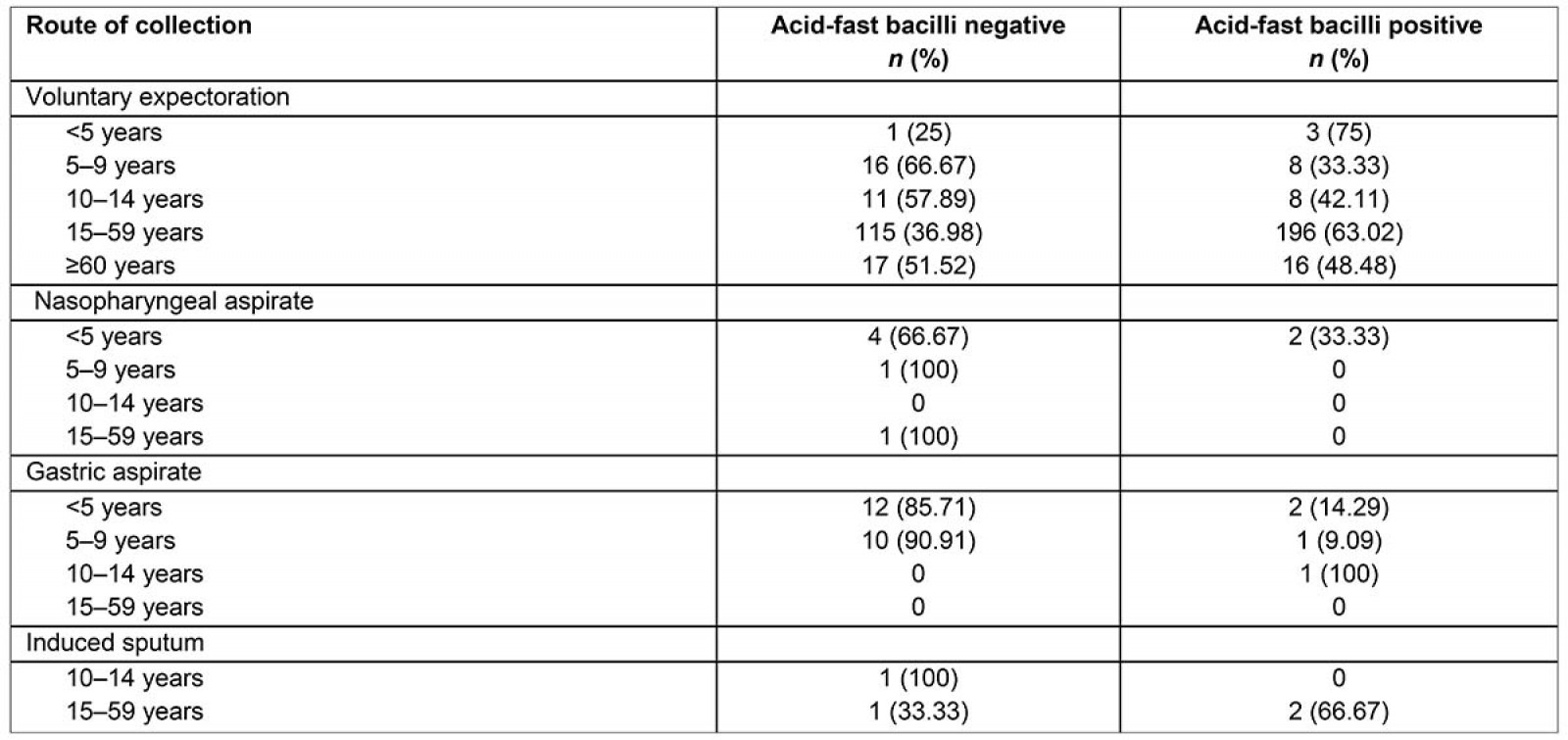
Table 4: Multivariate analysis of associations with a positive acid-fast bacilli smear result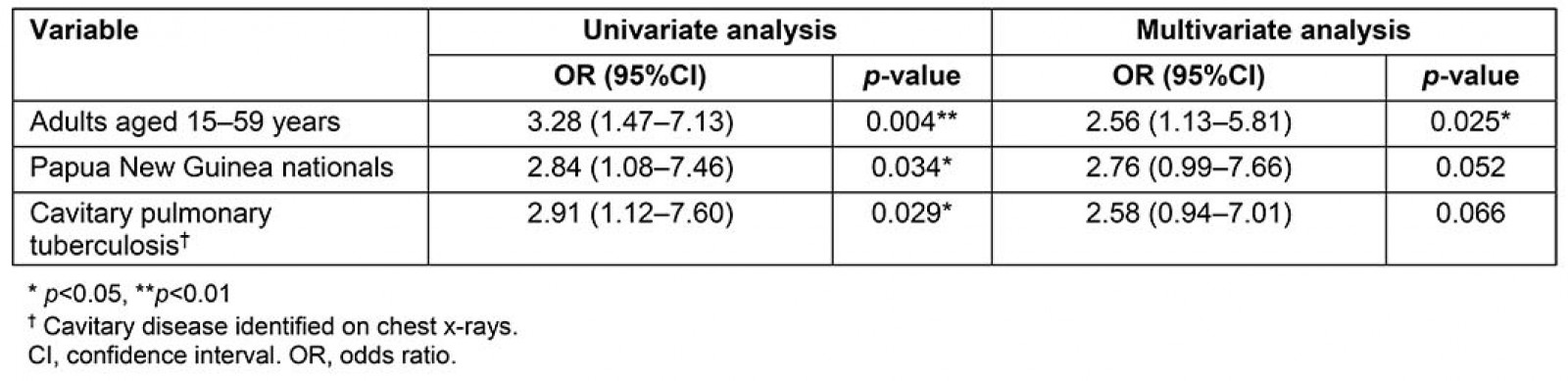
Discussion
This study demonstrated high sputum smear positivity among microbiologically confirmed cases, which suggests that many patients presented late for diagnosis. This was particularly true for PNG visitors who are not resident in the Torres Strait. Collection of a third sample for AFB microscopy had very poor incremental diagnostic yield and only served to unnecessarily delay treatment initiation.
As demonstrated in a univariate analysis, smear-positive PTB patients across all age groups were significantly (p<0.05) more likely to have cavitary disease, which contributes to a high bacillary load in sputum, and relapse and treatment failure among PTB patients17,18. Molecular epidemiological studies estimate that 13–20% of PTB cases are smear-negative19. While not as infectious as AFB smear-positive PTB, AFB smear-negative PTB can still be a source of transmission19.
Transmission risk is an omnipresent feature of TB control activities in the Torres Strait. Although physically located in Australia, the local TB program sits on the cusp of Australia and PNG. Delivery of TB services are atypical of a standard health system in terms of access, resources and ability to recall patients across an international border. With limited opportunity to retain patients in care, it is important for microscopy and culture to perform consistently well. Although the collection of three sputum specimens increases sensitivity of smear microscopy, microscopy is also prone to false-negative AFB smear-negative results and may fail to detect TB in children and in those at risk of dying from TB20.
Diagnostic yield is not solely reliant on the number of sputum specimens collected. In the Torres Strait Islands, TB diagnostics and treatment delays can be attributed to inclement weather, transport inefficiencies, distance between point of collection and reference laboratories, human resource and supply chain constraints, and test reliability issues due to contaminated specimens12. Most of these factors could be circumvented by incorporating advanced molecular technology in the region.
Without local access to the best diagnostic equipment – Xpert Ultra – there is a risk that MDR-TB cases will be missed. In an active TB case finding study conducted in Vietnam, clinicians diagnosed 41% of TB cases using Xpert that were otherwise culture-negative21. Diagnostic facilities in the Torres Strait are currently absent but increasing local diagnostic capability has the potential to alleviate transmission risk and the public health burden of PTB on both sides of the international border. With its capability to diagnose TB within 2 hours, strategic placement of the Xpert Ultra in the Torres Strait should be considered to reduce a serious public health risk. Benefits include ability to rapidly diagnose the most infectious cases. Further, the Xpert assay has a sensitivity of 73% for culture-confirmed AFB smear-negative PTB cases, which would reduce the time to treat22.
Clinician involvement in quality, collection modality, labelling errors, packaging and transport of specimens
This study confirmed that sample quality increases the potential for diagnostic yield and decreases the likelihood of missed cases. Further, an important component of AFB microscopy is that the level of infectivity of the patient is reported with smear-positive results. The smear-positive diagnostic yield for good quality specimens in this study was 96% within the first two specimens collected. Further, 96% (43/45) of poor quality specimens yielded a smear-positive result within the first two specimens collected. This suggests that poor quality should not be a contraindication for AFB microscopy; however, there is room for improvement in striving for quality specimens, which may lead to a better diagnostic yield.
In this study, 3 (0.7%) samples were not tested as they were considered too old, which is defined as samples received in the laboratory more than 7 days post-collection. The viability of sputum specimens for microscopy and culture also requires samples to be free of contamination by environmental or commensal bacteria that can inhibit the growth of M. tuberculosis23. The high humidity in the Torres Strait, in addition to more than 2000 km between collection points and reference laboratory settings, increase the likelihood that sputum specimens will be contaminated. A study conducted in Balimo in the Western Province of PNG reported TB case detection difficulties due to substandard storage and transport of specimens, which can adversely affect the viability of organisms24. Provision of free mandatory training in packaging and transport of specimens and adherence to pathology collection policies and procedures may help to reduce the numbers of ‘no-tests’ and potentially increase the diagnostic yield.
Voluntary expectoration is the preferred collection method in most passive case finding settings as the modality is cost-neutral and it is the only non-invasive approach. In this study, 91% of samples were voluntarily expectorated and were almost six times more likely to yield a smear-positive PTB result (p<0.001). It is unusual for young children to voluntarily expectorate; however, 28 children aged less than 10 years in this study were diagnosed with culture-confirmed PTB as a result of expectorated sputum. Of children aged less than 5 years, three (75%) were diagnosed with smear-positive PTB, and in children aged 5–9 years, one third were diagnosed with smear-positive PTB. Excessive exposure to woodfire smoke can cause a productive cough25, and while PNG villagers frequently use woodfires for cooking, it is unknown what effect smoke inhalation had on the young children in this study.
Where voluntary expectoration is not possible, sputum induction may be an optimal choice in primary healthcare settings in the Torres Strait Islands; however, it was not frequently used during the study period and a larger study would be required to draw such conclusions. A South African study of 250 children aged 1 month to 5 years reported that specimens were easily obtained from 95% of participants and that the diagnostic yield of sputum induction followed by extraction via nasopharyngeal aspiration was superior to three gastric lavage samples26. When considering age-related diagnostic yield across all collection modality types, 93% of children aged less than 15 years tested AFB-positive within the first two specimens collected. A shift in clinical practice and training provision merits consideration to increase the diagnostic yield for both adults and paediatric patients.
Conclusion
Reducing the number of specimens collected will reduce unnecessary delays in treatment initiation and increase retention in care for both Torres Strait Islander and PNG PTB patients accessing health services in the TSPZ. Limiting the numbers of sputum specimens to two per patient, provided they are quality specimens, should not compromise the diagnostic yield. In patients with two negative specimens, a third should be considered in those with persistent symptoms; however, this will only be possible for Australian residents, since patient recall is not possible across the existing international border.
Improving diagnostic yield in children should be a key feature of future TB strategic plans in this remote region. Although the rate of smear positivity was impressive in children in this study, it suggests that many children with sputum smear-negative disease may have been missed. Workforce training and development using a combination of collection modalities, as well as ready access to Xpert Ultra, is critical to increase diagnostic yield in paediatric patients across the age range.
In the Torres Strait and indeed all TB programs globally, the primary goal is to identify infectious PTB patients to break the transmission cycle. With high rates of sputum smear-negative PTB diagnosed in the Torres Strait Islands, coupled with difficulties in specimen collection and extended delays in getting AFB results in the region, this study has demonstrated a need to improve diagnostic sensitivity and reduce the time to treatment initiation. Collecting only two quality sputum specimens and having ready access to Xpert Ultra testing should achieve both aims, together with rapid identification of drug-resistant TB, which is a challenge in this setting7.
references:
You might also be interested in:
2022 - Social support in rural communities in Manabi province, Ecuador
2005 - Recruiting undergraduates to rural practice: what the students can tell us

