Introduction
Anemia is a pathological condition in which the number of red blood cells or the hemoglobin concentration within them is lower than normal. Although the global prevalence of anemia has decreased from 1990 to 2010, anemia is still a global public health problem that affects children under 5 years1. Worldwide, according to the WHO, 41.6% of children under 5 years were anemic in 20162. The most common cause of anemia in this age group is iron deficiency3, which if chronic will lead to impaired development, poor cognitive function and, ultimately, fewer opportunities4. Other causes include infections, chronic diseases and malnutrition5. Moreover, increased mortality has been linked to anemia in children under 13 years6.
Anemia is still a public health issue as established by the Sustainable Development Goals7. Among Latin American and Caribbean countries, the overall prevalence of anemia in children under 5 years was 28.4% in 20162. In Peru, despite the reduction in the prevalence of anemia from 37.7% in 2010 to 32.8% in 2018, anemia remains a major public health problem8. Nationwide, the 2017–2021 National Plan for the reduction and control of maternal and infant anemia and chronic infant malnutrition was implemented by the Ministry of Health. Its purpose was to reduce the prevalence of anemia in children aged 6–36 months to 19% by 2021 and the prevalence of chronic malnutrition in children under 5 years to 6.4% by 20219. Additionally, several programs have been developed over the years to tackle anemia prevalence in children10.
Anemia remains a public health concern, especially in high-risk zones such as rural areas. Peruvian children living in rural regions are the most affected, with a prevalence of anemia of 39.6%, compared to 30.2% of anemic children living in urban areas in 20188. Several sociodemographic factors have been associated with anemia in children11-14. However, components contributing to the urban–rural gap have not been previously assessed. Factors such as nutrition status, mother´s education level, wealth index and access to healthcare services may be associated with that disparity15,16. Other factors might explain this gap, such as parasitic infections and environmental exposures17,18. The application of innovative methods may allow determination of the main factors contributing to the urban–rural gap in childhood anemia.
Given the public health relevance of anemia, it is important to assess its geographic distribution, which could help to implement targeted interventions in areas with high prevalence. In addition, identifying factors that explain the difference in anemia between rural and urban areas may serve to carry out programs addressing these variables, thereby reducing this gap and the burden of the disease. The purpose of this study was to evaluate the determinants of the difference in anemia prevalence between urban and rural areas, and the spatial distribution of anemia in Peruvian children aged 6–59 months.
Methods
Study design and data source
A secondary data analysis was conducted using the 2019 Peruvian Demographic Health Survey (DHS), a national survey executed by the National Institute of Statistic and Informatics (INEI). Peru is an upper-middle income country in western South America, with a population of approximately 32 million people. The survey design was probabilistic, multistage, stratified, balanced and independent at the departmental level (24 departments and 1 constitutional province) and location of residence (urban and rural). It includes national, regional and residential area representativeness. Administratively, Peru is divided into 24 departments and 1 constitutional province, which are subdivided into provinces, districts and population centers. In the DHS database (http://iinei.inei.gob.pe/microdatos), the urban sampling unit comprises conglomerate and private housing, while the rural sampling unit comprises rural registration areas and private housing. Although DHS data is collected at the household level, spatial data is located at the sampling cluster level. The sample size of the 2019 DHS was 36 760 households: 24 100 from urban areas and 12 660 from rural areas19. The study population included children aged 6–59 months, who had their hemoglobin level measured and adjusted for altitude. Records with incomplete data for any variables of interest were excluded.
Outcome variable
Hemoglobin concentration was measured using the HemoCue® Hb201 portable hemoglobinometer. Its measurement was based on the modified azide–methemoglobin reaction. This method is recommended by the WHO when conducting large population studies. The hemoglobin concentration was adjusted by altitude using the following equation designed by the US Centers for Disease Control and Prevention:
Hbcorrected = Hbmeasured – altitude adjustment
where altitude adjustment = –0.032 × MASL (meters above sea level) + 0.022 × MASL
A hemoglobin concentration less than 11 g/dL was considered to be anemia. This technique has a sensitivity of 85% and a specificity of 94%. In Figure 1, anemia was categorized using WHO recommendations for severe (<7 g/dL), moderate (7.0–9.9 g/dL) and mild (10.0–10.9 g/dL)20-22.
Independent variables
The independent variables were wealth index, mother’s education level, father’s education level, mother’s age, number of children, place of birth and mother’s employment status. The wealth index is a socioeconomic measure comprising living assets of the household interviewed. For example, it includes household ownership over selected holdings such as bicycles, automobiles, home appliances, access to basic services, access to healthcare services, among others. It is calculated through a statistical technique known as principal components analysis. It separates all households into five wealth quintiles: poorest, poor, middle, rich, richest. It enables statistical procedures to be applied in order to establish the impact of this measure over various demographic indicators23. Mother’s and father’s education level were categorized into no education or primary, secondary and higher. Mother’s age was categorized into 15–24 years, 25–34 years and 35–49 years. Number of children was dichotomized into less than five and five or more children. Place of delivery was dichotomized into home and health facility delivery. Mother’s employment status was dichotomized into employed and unemployed.
Data management and analysis
The database was downloaded in .dbf format from the webpage of INEI and imported into STATA v16.0 (StataCorp; http://www.stata.com). Then, the databases of the questionnaires that included the variables of interest were merged. The analysis was performed taking into account the complex design of the survey to reduce sampling errors. It was weighted using sampling weight, primary sampling unit and strata. P-values less than 0.05 were considered significant and confidence intervals were calculated to 95% (95%CI).
Multivariate decomposition analysis
This study employed the multivariate decomposition for non-linear response models to determine the major factors contributing to the difference in the prevalence of anemia between urban and rural areas. Several studies have applied this technique to identify factors associated with change over time and to determine factors associated with differences between two groups24-26. In the present study, the purpose of this analysis was to identify the source of differences in anemia prevalence between rural and urban areas. This technique uses the output of a regression model to divide a difference between groups into two components (overall decomposition). The logit-based multivariate decomposition was used. For logistic regression, the logit or log-odd of anemia prevalence across residence areas is represented as:
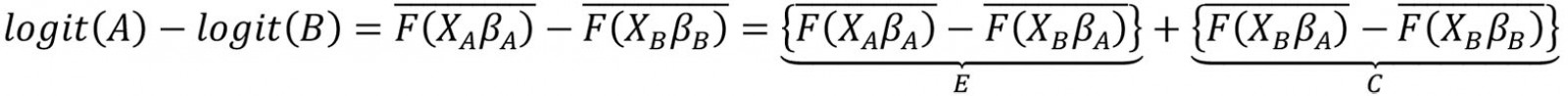
The E component represents the part of the differential attributable to differences in characteristics or endowments (characteristics effects) of the population, and the C component represents the differential attributable to differences in coefficients or effects of the explanatory variables (coefficients effects). After that, E and C were separated into portions in order to estimate the contribution of each factor to each component (detailed decomposition). This analysis was done through the mvdcmp command27.
Spatial analysis
The spatial analysis was performed using ArcGIS version v10.8 (Esri; http://www.arcgis.com) and SaTScan v9.6 (SaTScan; http://www.satscan.org). First, the prevalence of anemia was calculated for each Peruvian region and plotted into a choropleth map. Global Moran's I was then employed to measure spatial autocorrelation. Moran's I ranges from –1 to 1. A positive value indicates a clustered spatial pattern, 0 indicates a randomly distributed pattern and a negative value indicates a dispersed pattern. Ordinary Kriging spatial interpolation method was then used to predict the prevalence of anemia in those unsampled regions based on the sampled regions.
Kulldorff's purely spatial scan statistic based on the Bernoulli model was used to identify high-risk anemia clusters. It uses a circular window that moves across the area under study to identify clusters. Children with anemia were deemed as cases and those without anemia were deemed as controls to fit the Bernoulli model. The maximum spatial size of the clusters was configured at <5% of the population as the upper limit, which allows identification of small clusters. For each potential cluster, a log-likelihood ratio test (LLR) was reckoned, which allowed determination of whether the observed number of cases within each potential cluster was significantly higher than expected or not. It was calculated based on 999 Monte Carlo replications. LLR and p-values were used to determine significant clusters. The primary cluster was defined as the one with the highest LLR as it was the most likely performing cluster. All clusters were ranked according to their LLR28.
Ethics approval
The protocol was reviewed and approved by the Institutional Review Board of Universidad Peruana de Ciencias Aplicadas (registration code PI081-17).
Results
In this study, a total weighted sample of 17 638 children aged 6–59 months with hemoglobin measurement and complete data for the variables of interest were included in the analysis (Supplementary figure 1).
Sociodemographic characteristics
Of all participants included in this analysis, 71.49% lived in urban areas. Concerning the wealth index, only 6.77% of children who lived in urban areas belonged to the poorest wealth quintile, while almost three-quarters (74.20%) of those who lived in rural areas belonged to the poorest wealth quintile. Regarding mother's education level, there was also a difference between urban and rural areas. From those who lived in urban areas, only 10.26% had a mother with primary or absent education; this percentage increased to 47.07% in those from rural areas. A similar figure was observed for father's education level. Regarding number of living children, 17.62% who lived in rural areas had a mother who reported having five or more living children, compared to 5.64% who lived in urban areas. From those who lived in rural areas, 28.92% had a mother aged 15–24 years; this percentage decreased to 20.47% in those from urban areas. Concerning the place of delivery, 20.06% of children who lived in rural areas were born at home, compared to those who lived in urban areas where only 1.27% were born at home (Table 1).
Table 1: Characteristics of children aged 6–59 months, urban and rural areas of Peru, 2019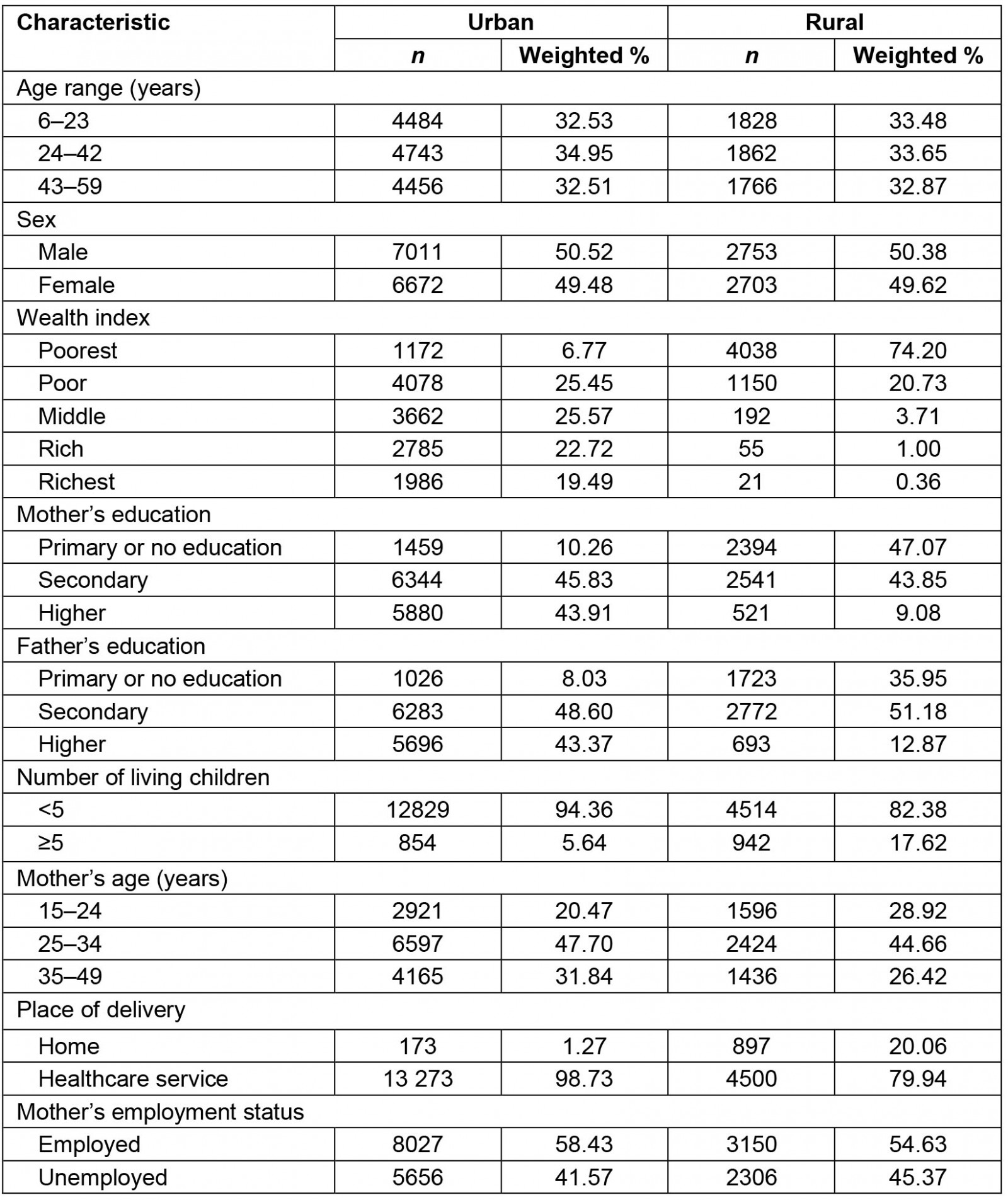
Prevalence of anemia
Nationwide, the prevalence of anemia was 29.47% (95%CI 28.63–30.33). In rural areas, the prevalence was 38.25% (95%CI 36.53–39.99), whereas in urban areas it was 26.39% (95%CI 25.44–27.37). This is a gap of 11.86 percentage points between the prevalence of anemia in rural and urban areas. In urban areas, 19.23% had mild anemia, 7.09% had moderate anemia and 0.06% had severe anemia; in rural areas, 26.17% had mild anemia, 11.89% had moderate anemia and 0.18% had severe anemia (Fig1). The regions with the highest prevalence of anemia were Puno (59.61%), Cusco (46.37%) and Huancavelica (45.23%). Regions with the lowest prevalence of anemia were Cajamarca (20.3%), Lima (22.24%) and La Libertad (22.81%) (Fig2).
The prevalence of anemia varied according to the sociodemographic characteristics of children aged 6–59 months. The difference in anemia between children living in rural areas and those living in urban areas was always positive. Generally, a higher prevalence of anemia was found in children living in rural areas compared to those living in urban areas (Table 2).
Table 2: Anemia distribution in children aged 6–59 months, urban and rural areas of Peru, 2019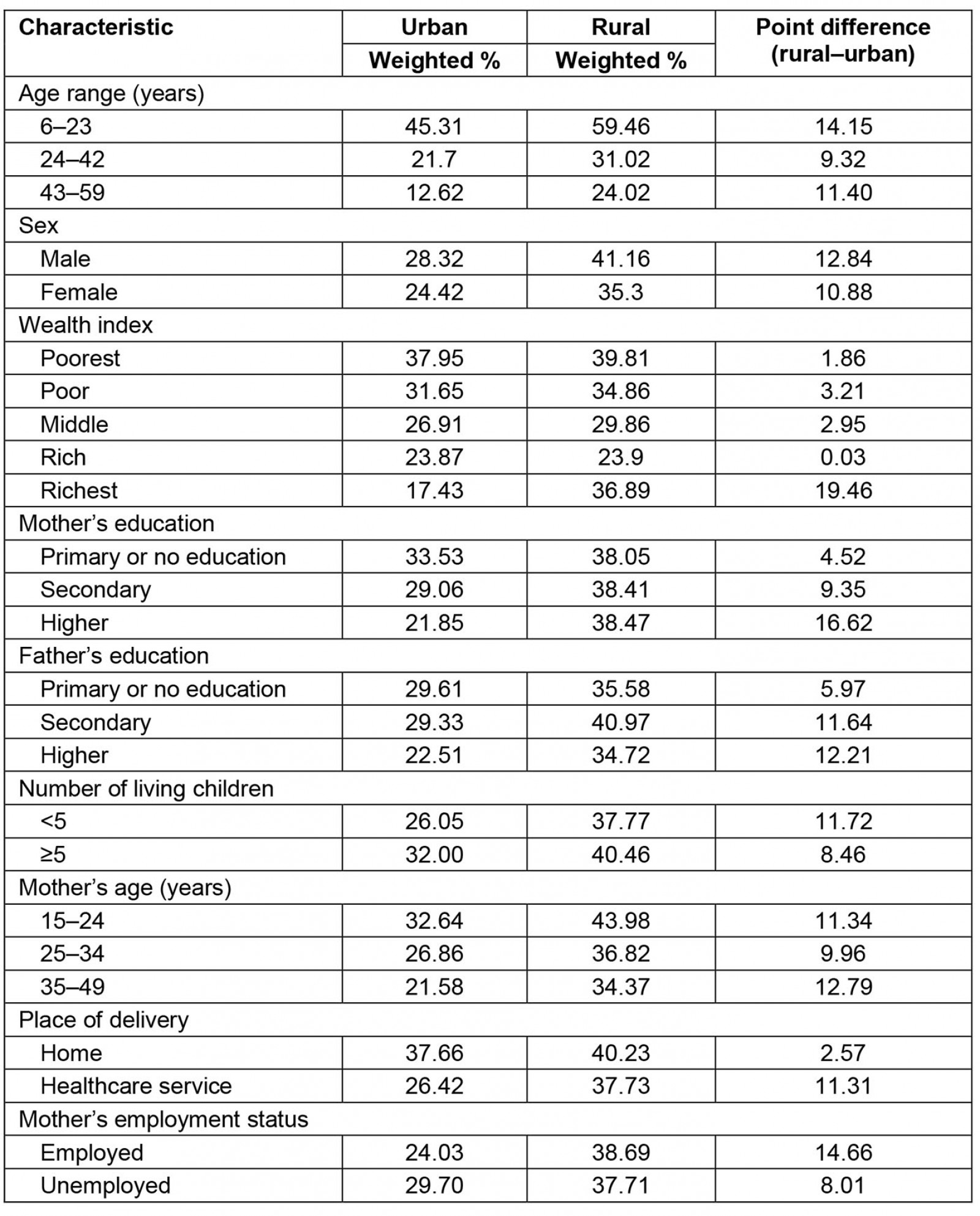
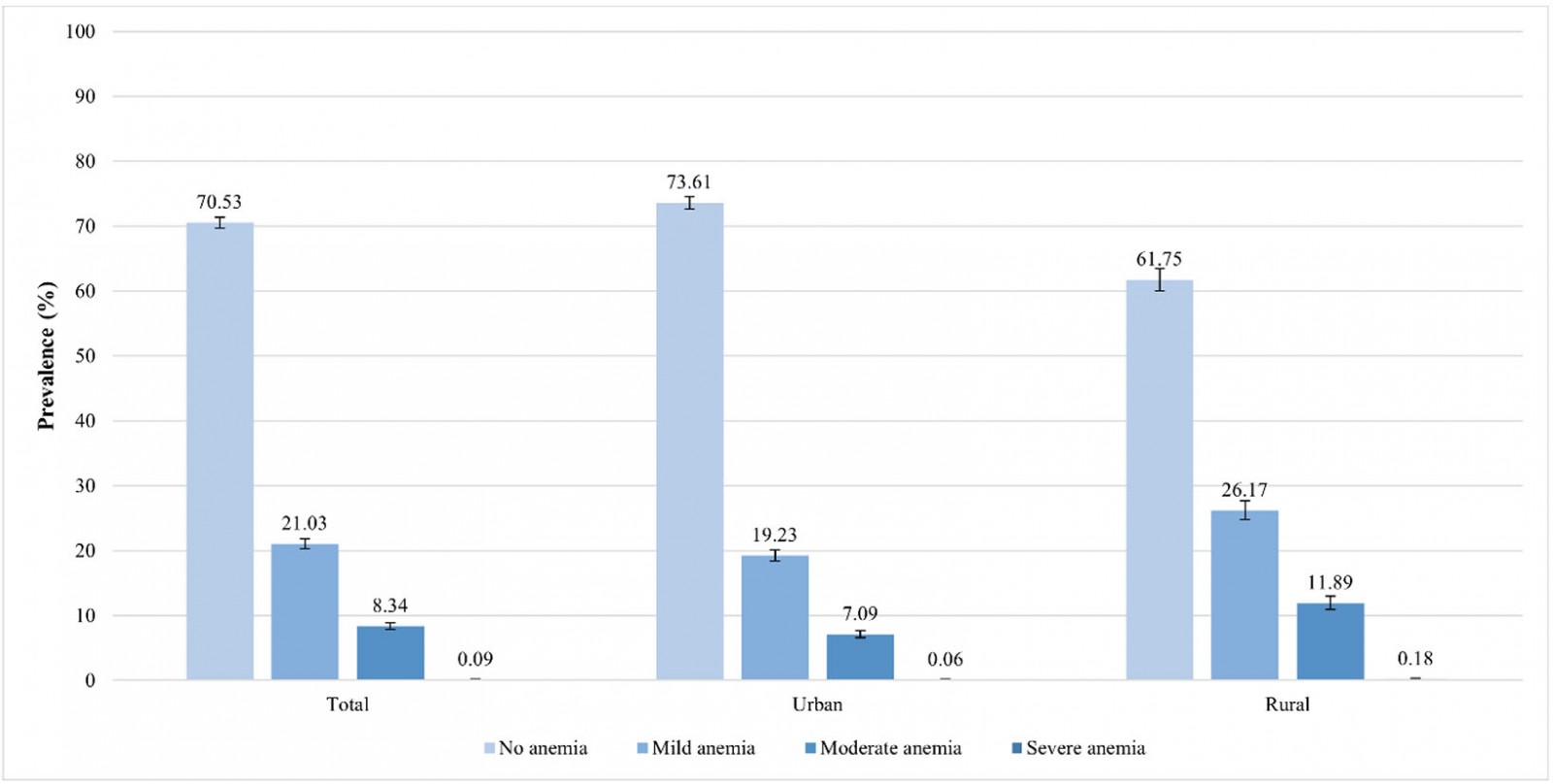 Figure 1: Anemia severity among urban and rural areas in children aged 6–59 months, Peru, 2019.
Figure 1: Anemia severity among urban and rural areas in children aged 6–59 months, Peru, 2019.
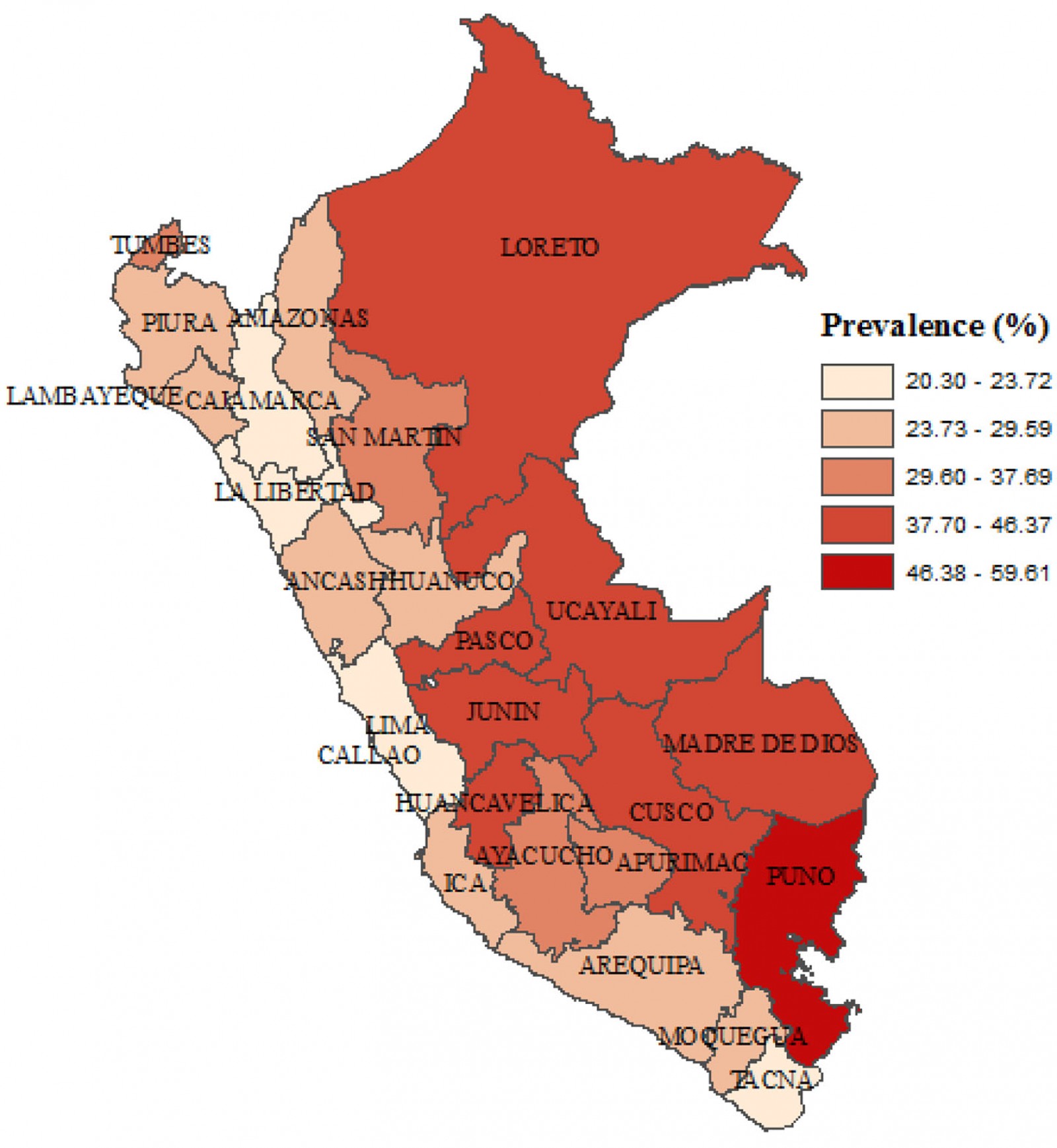 Figure 2: Choropletic map of anemia in children aged 6–59 months at the departmental level, Peru, 2019.
Figure 2: Choropletic map of anemia in children aged 6–59 months at the departmental level, Peru, 2019.
Decomposition analysis
The decomposition analysis revealed that 88.61% of the difference in the prevalence of anemia between urban and rural areas was attributed to the difference in respondent's characteristics. This was explained by the difference of the distribution in the wealth index, the mother's education, the number of living children, the mother's age and the mother's labor status. More than 1%, 14%, 19% and 27% of the differences in anemia prevalence were explained by the poor (β=–0.002; 95%CI: –0.004– –0.0005), middle (β=–0.018; 95%CI –0.027– –0.008), rich (β=–0.023; 95%CI –0.032, –0.013) and richest (β=–0.033; 95%CI –0.043– –0.023) wealth index across residential areas, respectively. Higher mother´s education significantly contributed to the difference in anemia prevalence by 13.45% (β=–0.016; 95%CI –0.031–0.002). Mother's employment status (β=–0.001; 95%CI –0.002,0.0005) and having fewer than five living children (β=–0.005; 95%CI –0.010–0.0002) among urban residents significantly contributed to the difference in anemia prevalence roughly by 1 and 4%, respectively. Mothers aged 15–24 years (β=–0.0009; 95%CI –0.002– –0.0003) and those aged 35–49 years (β=–0.005; 95%CI –0.007–0.004) significantly contributed, by 0.76% and 4.39%, respectively, to the differences in anemia prevalence (Tables 3,4).
Table 3: Overall decomposition analysis of anemia between urban and rural areas in children aged 6–59 months, Peru, 2019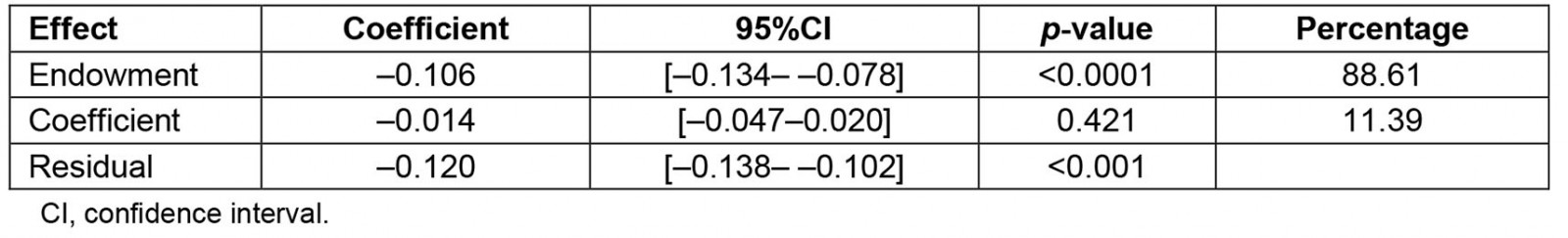
Table 4: Detailed decomposition analysis of anemia between urban and rural areas in children aged 6–59 months, Peru, 2019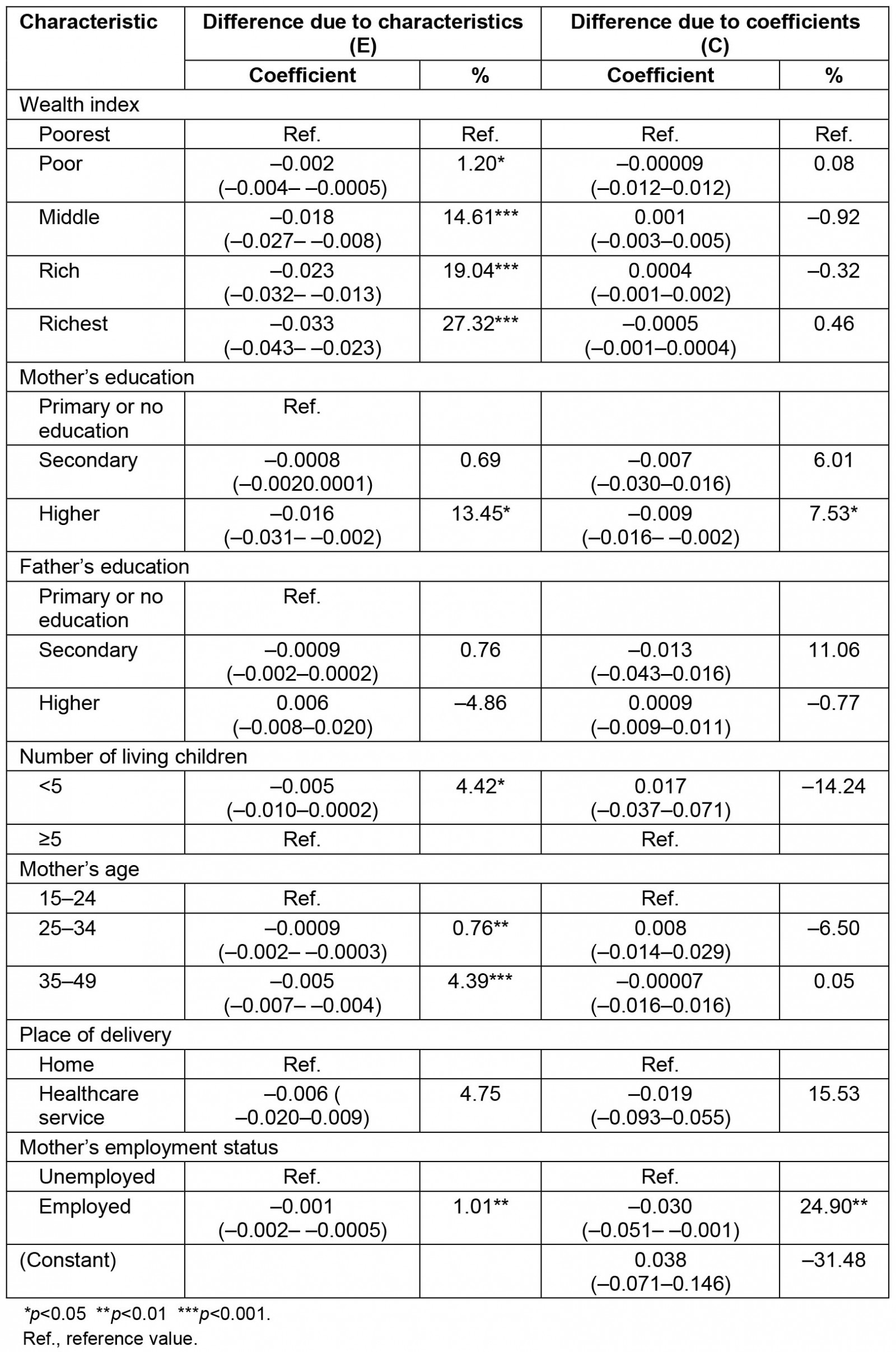
Spatial analysis
The pattern of the spatial distribution of anemia in children was clustered (Moran´s Global I autocorrelation 0.04, p<0.0001) (Fig3).
Kriging's interpolation estimated that the spatial distribution of the prevalence of anemia is heterogeneous at the inter- and intradepartmental level. The regions located in the center, south-east and north-east of Peru had the highest prevalence of anemia. These regions include Puno, Loreto, Amazonas, Cusco, Madre de Dios and Ucayali. The regions located at the coast had the lowest prevalence of anemia (Fig4).
The SaTScan spatial analysis detected 1003 clusters of areas with high prevalence of anemia, of which six were statistically significant. The most likely primary SaTScan cluster of high prevalence of anemia was located in the south-east of Peru, mainly in the Puno region (LLR=127.76, p<0.0001). The second most likely cluster (LLR=37.90, p<0.0001) was located at the center of the country and encompasses Junin and Huancavelica region. Similarly, the third most likely cluster (LLR=35.51, p<0.0001) was located in the center of Peru, mostly in the north of Lima. The fourth (LLR=27.56, p<0.0001) and fifth (LLR=21.79, p<0.0001) most likely clusters were located in the north-east of Peru, predominantly in the regions of Loreto and Ucayali. The sixth most likely SaTScan cluster (LLR=12.61, p<0.0001) was located in the south of Peru, principally in the region of Cusco (Table 5 and Fig5).
Table 5: Most likely SaTScan clusters of areas with high prevalence of anemia among children aged 6–59 months, Peru, 2019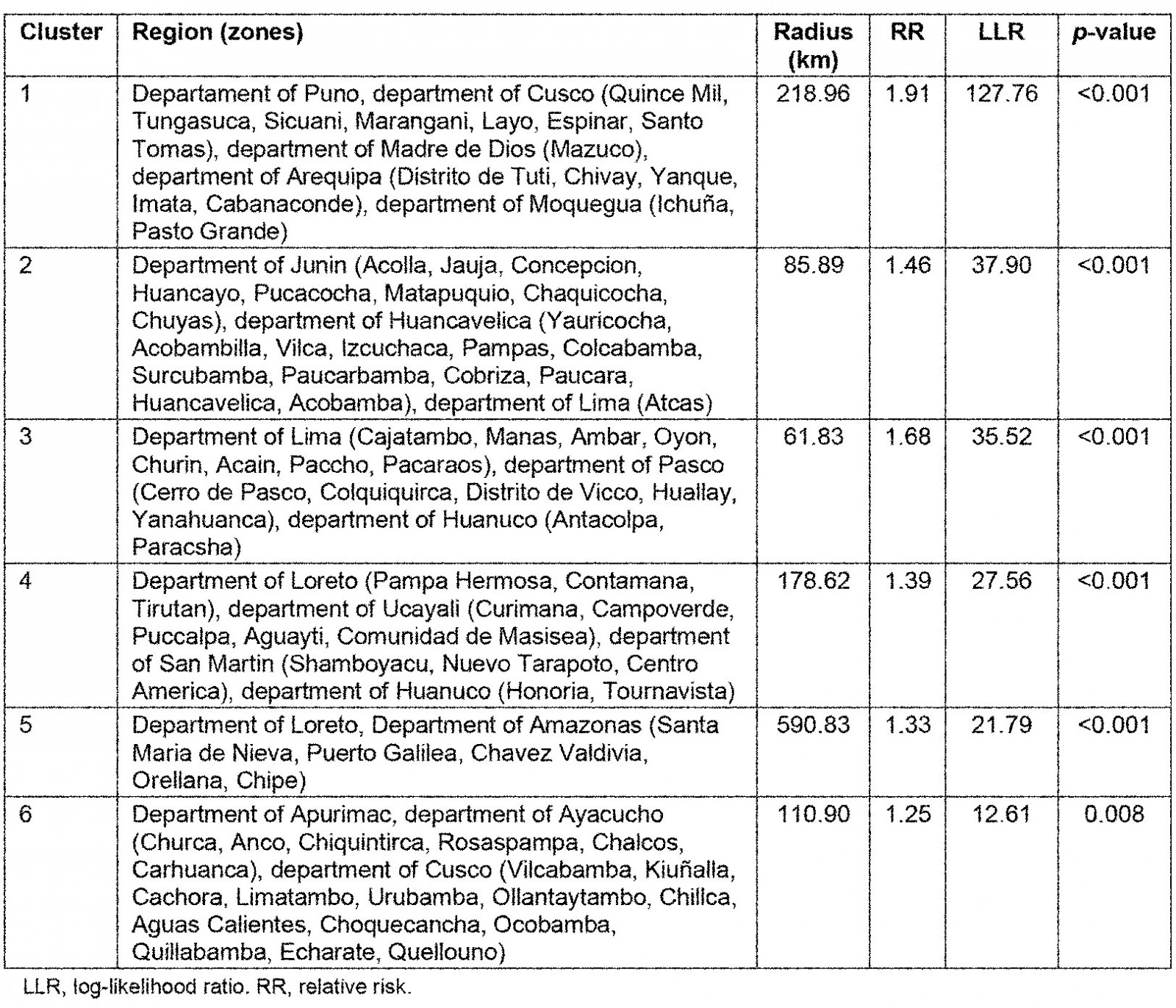
Figure 3: Global spatial autocorrelation of anemia in children aged 6–59 months, Peru, 2019.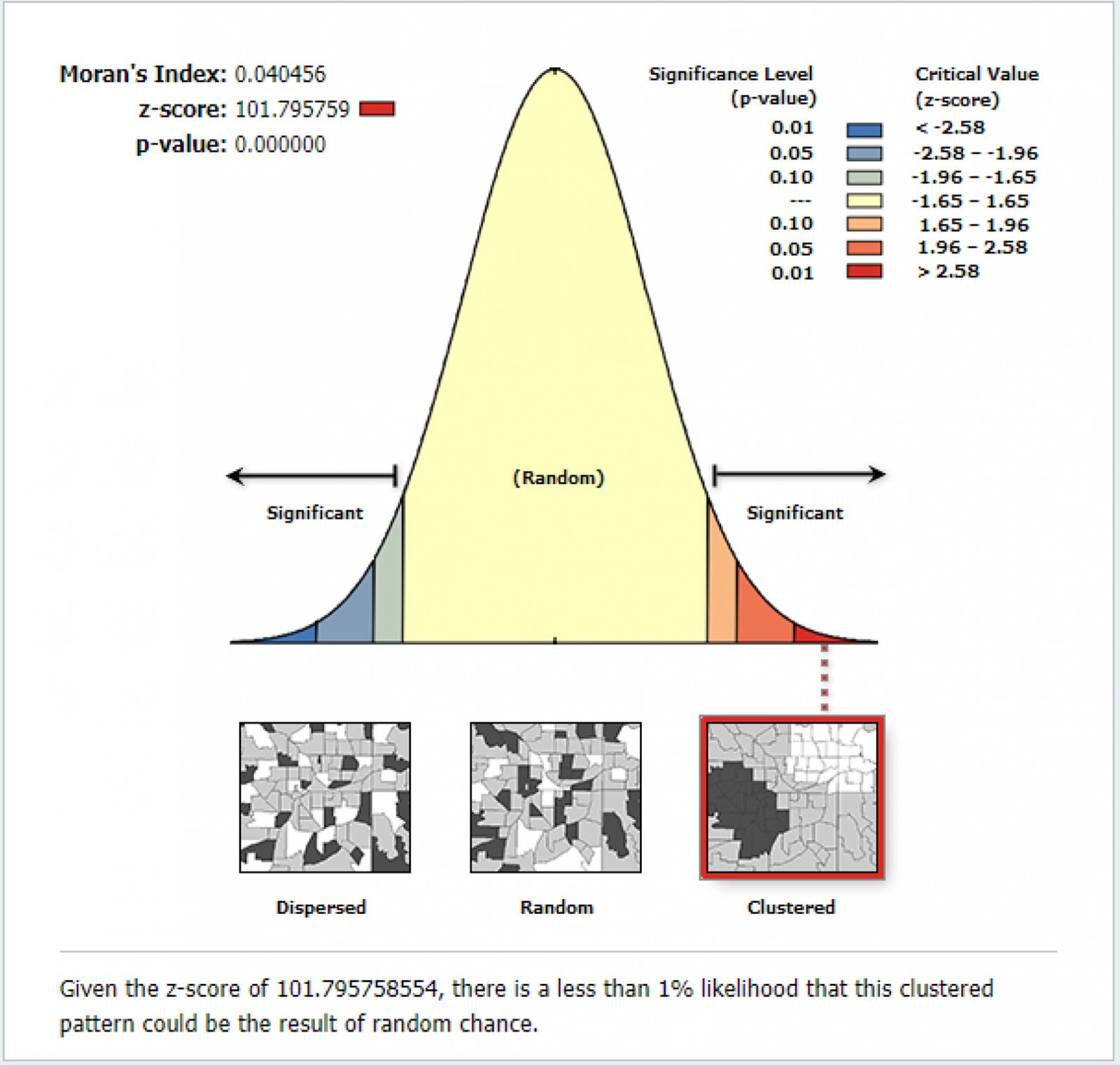
Figure 4: Kriging interpolation of anemia in children aged 6–59 months, Peru, 2019.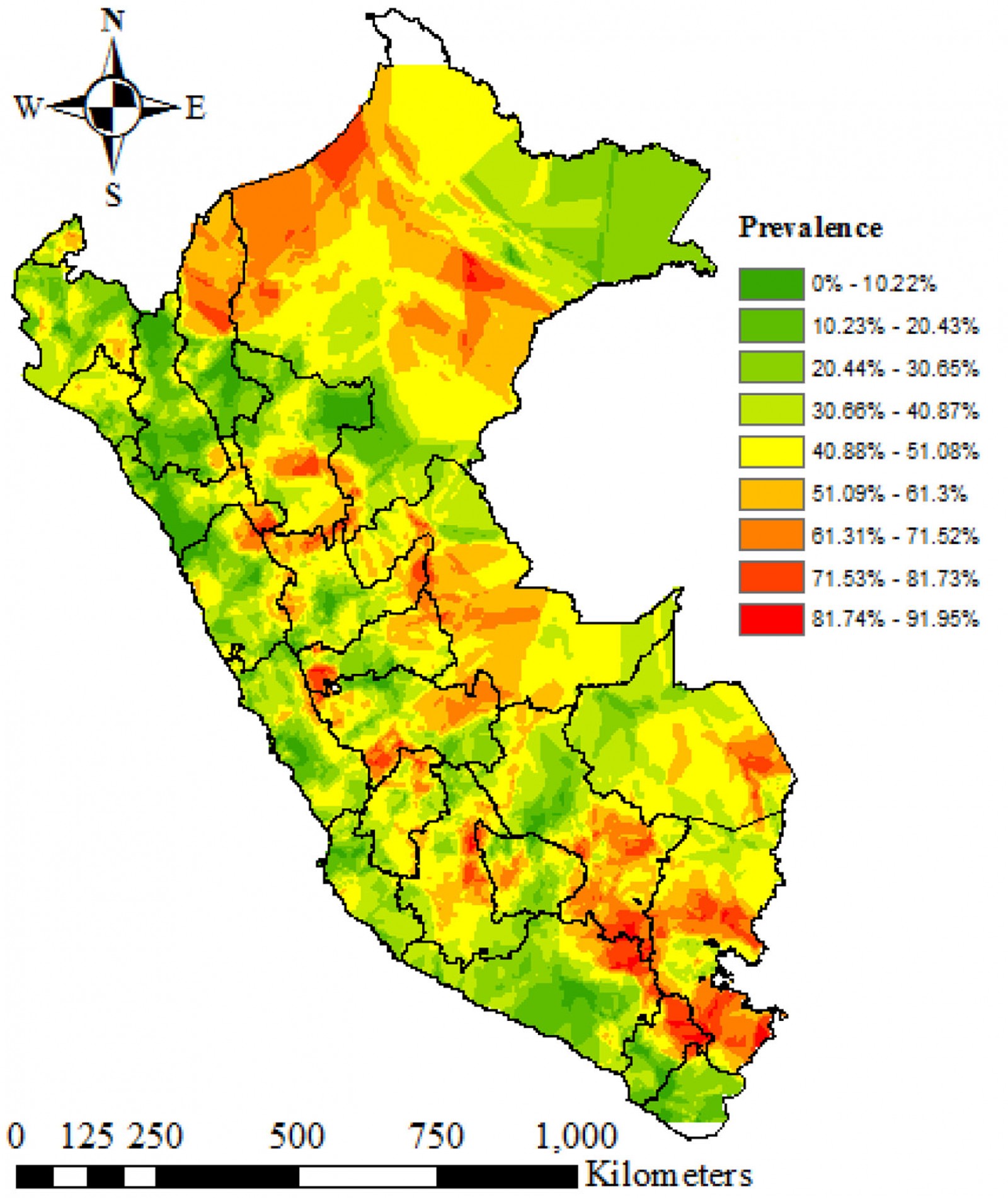
Figure 5: SaTScan Analysis of areas with high prevalence of anemia in children aged 6–59 months, Peru, 2019.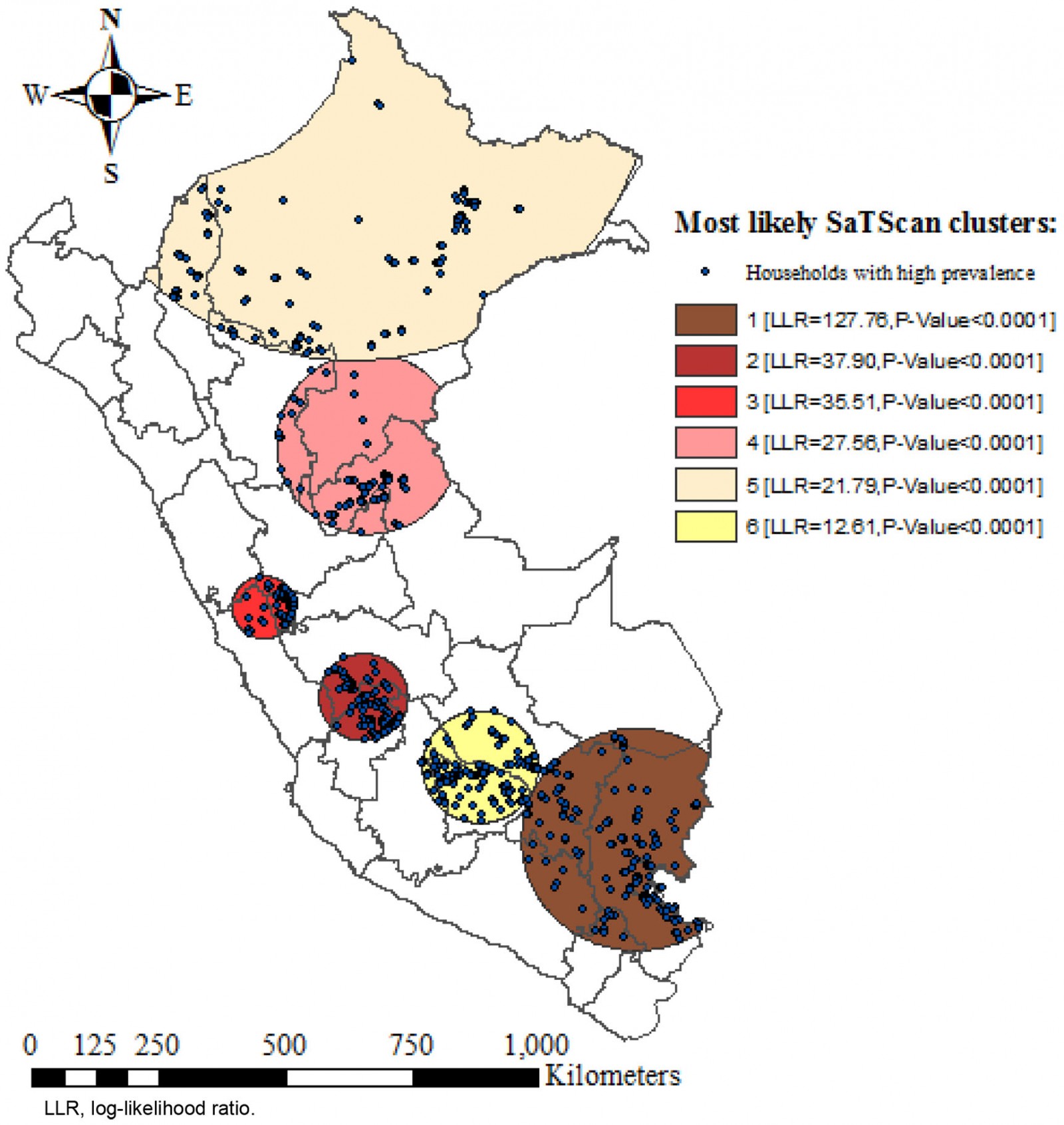
Discussion
To the authors’ knowledge, there is no other study in South America evaluating anemia in children aged 6–59 months through multivariate decomposition analysis. Nationwide, the prevalence of anemia is 29.57%, principally driven by rural areas. Puno (59.81%) and Cusco (46.37%) were the regions with the highest prevalence of anemia, while La Libertad (22.81%) and Lima (22.24%) were the regions with the lowest prevalence. Likewise, there is a wide difference (11.86%) in prevalence between urban and rural areas. The decomposition analysis showed that 88.61% of the difference in the prevalence of anemia between urban and rural areas was attributed to the difference in the composition of the respondents’ characteristics between these areas. Wealth index, a higher mother’s education, fewer than five living children, employed mother and mother’s age were significant predictors of the difference in the anemia prevalence between areas of residence. Some previous studies have assessed childhood anemia both in urban and rural areas through multivariable regression models for each area; multivariate decomposition integrates both areas in one statistical model27,29.
A previous spatial analysis on anemia in Peruvian children was conducted by Hernandez et al15. Nevertheless, it is not recent and raises some methodological concerns. First, the researchers used only empirical spatial analysis techniques, which prevented them from providing an adequate evaluation of the whole problem. The localization of each cluster was only based on the measurement of local Moran´s I. Second, the study did not provide an exhaustive characterization of each cluster. Third, the lack of a standardized method to measure hemoglobin levels impeded consideration of both the sensitivity and the specificity of the estimates.
In comparison, the present study provides a comprehensive characterization of each cluster, including a table with the districts embedded in each of them. Additionally, Ordinary Kriging interpolation estimated the prevalence in each region. Herein, a heterogeneous spatial distribution of the prevalence of anemia across the area of study was observed, although clustered spatial pattern was found. Six clusters of areas with high prevalence of anemia were detected in the north-east, center and south regions of Peru. The estimates were clearly standardized, and both sensitivity and specificity were adequate, especially for epidemiological studies.
According to the present study results, a prevalence of anemia of 29.57% was found, which is above the prevalence of anemia in Latin American and Caribbean countries, which is 28.42%2. Moreover, according to previous systematic reviews, anemia prevalence in Peruvian children is higher than in neighboring countries such as Chile (4.0%) and Argentina (7.6%); however, it is below Brazil (40.2%) and Bolivia (61.3%)30,31. Despite different strategies put in place to prevent anemia, such as late umbilical cord clamping, fortified food consumption, deworming and iron supplementation in children, teens and pregnant women32, Peru is still among the countries with the highest prevalence of anemia in Latin American and Caribbean countries30.
Nationwide, the Ministry of Health implemented the 2017–2021 National Plan for reduction and control of maternal and infant anemia and chronic infant malnutrition. Its purpose consisted of reduction of the prevalence of anemia to 19% in children aged 6–36 months by 20219, which contrasts significantly with the prevalence of anemia found in the present study. Additionally, several social programs and interventions on anemia have been developed in the last years to address this problem. Examples are the Articulated National Program (PAN), the Chispitas (Sparkles – Nutriwawa) initiative, and the Program of Educational Intervention in Prevention of Anaemia and Undernutrition in Pachacutec-Ventanilla10. The last one focused on the improvement of the nutritional status of children aged less than 5 years and pregnant women through empowerment of education and health care, food supplementation and strengthening of social organizations33. Project’s results showed a reduction in 70% of anemia in children aged less than 5 years, and after 2 years of intervention an adequate food intake was maintained in 98% of these children10. These results elicit its potential for replicability through different latitudes in Peru. The implementation of family-centered programs and policies, such as conditional cash transfer and incentives for rural business, is necessary in rural areas.
Food security must be guaranteed in both rural and urban areas especially among high-risk populations (children and pregnant women). In this sense, food-based interventions, such as supplementation and fortification, are crucial to tackle geospatial disparities of anemia in childhood. Regarding supplementation, it is not only important to provide oral iron supplements, but also other vital micronutrients to improve iron absorption (such as vitamin C), iron metabolism (such as vitamin A) and hematopoiesis (such as folic acid and vitamin B12)34. Also, biofortification of foods with iron and vitamin A can be considered. A food already employed for biofortification with these nutrients is rice35.
Public health interventions designed to target family planning and nutrition education at primary care level, particularly directed towards mothers, would be helpful, desirable and long-lasting. These interventions must include the increased intake of iron-rich foods, avoidance of dietary inhibitors (such as phytates, tannins and oxalates), consumption of enhancers of iron absorption (such as citrus) and improvements on the processing, preservation and preparation techniques of iron-rich meals36. Nevertheless, the measurement of the impact of these interventions can take a long time.
The multivariate decomposition analysis showed that wealth index significantly contributed to the difference in anemia prevalence between rural and urban areas. A higher contribution to this difference was observed as wealth index increased. This is widely supported by literature11,37,38. A study performed in Ethiopia described that children from families with a monthly income less than the minimum wage were more likely to have anemia14. Moreover, in another study, children aged less than 5 years were less likely to have anemia if their family wealth index was above the poorest index, which is consistent with the present study’s findings. Despite the WHO recommendations for a nutrient-rich diet, the poorest families may not be able to pay for diversified foods39. Additionally, the poorest families may have health access difficulties, which could lead to late anemia diagnosis and inadequate treatment13. Government policies must reinforce the distribution of fortified foods and/or supplements for rural families with children aged less than 5 years through multisectoral and intersectoral programs, especially in the high-prevalence clusters identified in the present study.
Maternal age significantly contributed to the difference in anemia according to place of residence. Additionally, the prevalence of anemia in children is higher at early maternal ages in both urban and rural areas, being greater in the latter. These results could be explained by maternal and perinatal complications of adolescent pregnancy itself. A study conducted in teenage mothers who were admitted to a rural hospital in west Bengal in India showed that anemia in the last trimester of pregnancy, low birth weight and preterm delivery were more frequent among those mothers aged 15–19 years in comparison to those mothers aged 20–24 years40. Similarly, a study conducted in the USA found that the risk of maternal anemia is higher in adolescent pregnancy, especially at younger ages (less than 16 years)41. Moreover, a systematic review concluded that premature and low birth weight infants are more prone to develop iron deficiency anemia42. A study conducted in four South African countries found that maternal anemia increases the odds of anemia in children43.
The difference in the prevalence of anemia in children according to residence area is primarily due to limited healthcare access, particularly in family planning and reproductive health services44. A study based on DHSs of five countries, including Peru, found a greater proportion of teenage pregnancy among rural residents and in the poorest populations45. Hence, reinforcement of family planning and reproductive health services in rural areas may reduce the gap of anemia in childhood according to place of residence.
According to the present study’s findings, having fewer than five children significantly contributed for the difference in anemia in childhood according to place of residence. In addition, a higher prevalence of anemia was found among those children whose mothers have had five or more children both in urban and rural areas, being higher in the latter. Similarly, a study conducted in children from rural Chad found that larger family size increases the odds of anemia in childhood46. The relation between anemia in children and high parity could be explained by maternal anemia. Multiparity has been found to induce maternal anemia by both reducing maternal iron reserves in each pregnancy and blood loss during birth47. Family size is consistently larger in rural areas, which may be explained by a greater desired and actual number of children48,49 and a low prevalence of modern contraceptive use50. This greater desired and actual number of children in rural areas may be driven by the fact that a larger family size implies more people to work on their lands51. A large family size has been associated with anemia in children, even when adjusting for multiple confounders52. When there are a higher number of individuals within a family, sharing foods may become a potential risk factor for iron deficiency anemia, especially when considering the evidence from diverse studies showing higher prices of foods with high protein content (particularly animal source foods)53. Conversely, smaller families would be related to a higher mother´s education status, which is linked with improvements in child health, even when health services are accessible54.
In the present study, mother’s education contributed to the difference in the prevalence according to place of residency. A prior study has indicated that there is a relationship between children nutritional status and higher maternal education55. Mother’s education plays an important role in nutritional decision making, because educated mothers are more likely to fulfill the micronutrient requirements of the child12. As a result, a higher maternal education status has a potential positive effect on the quality of a child’s diet and may reduce the risk of iron deficiency anemia56. A recent study conducted in Indonesia has shown that mothers’ knowledge of anemia and its symptoms is associated with lower odds of anemia in children aged 6–59 months in rural settings but not in children from urban areas. This result may be explained by higher consumption of animal source foods in rural families but not in urban families57. Furthermore, mother´s education is important, since it has been shown to improve complementary breastfeeding practices, which is a critical stage in a child´s development58. Therefore, the present study highlights the key role of improving maternal educational level, which may help to reduce the gap of the prevalence of anemia between urban and rural areas.
Mother’s employment status significantly contributed to the difference in the prevalence of anemia in Peruvian children living in rural and urban areas. This finding may be explained by the fact that unemployment may lead to a lower income and poor socioeconomic status, as mentioned in other study59. Consequently, families with suboptimal socioeconomic conditions may face barriers in accessing nutrition and healthcare services, which may result in a higher risk of anemia. Another viable explanation for this gap is that there are more job opportunities in urban areas, compared to rural settings in low- and middle-income countries60. Conversely, a recent study conducted in urban areas of Brazil showed a higher prevalence of anemia among children whose mothers had jobs, which could be explained by the lack of time to offer an adequate diet61. These implications suggest that residents of rural areas may have a unique set of features that place children's nutrition at a disadvantage, and children at risk of anemia.
Despite the heterogeneous distribution of the prevalence of anemia at both inter- and intra-departmental levels, six high-risk clusters of anemia were detected. The results obtained in the present study are quite similar to findings from a previous study conducted with data from 201615, which suggests that there could be unique and intrinsic factors that explain the persistence of a high prevalence of anemia in those areas. The departments located inside the clusters have the lowest human development index compared to other Peruvian departments62. Many studies have widely described that anemia highly affects poor socioeconomic groups, as already discussed15,38. Other contributors for spatial clustering of anemia are having anemic mothers, malnutrition and parasitic infections13,63. Schistosoma haematobium and malaria have been associated previously with the spatial variation in children´s anemia risk63. The former is absent in the Peruvian setting. Interestingly, the geographic disease-disease interaction between malaria and anemia has been widely described in the literature64. Malaria is endemic in the Amazon region, particularly in Loreto department65, where the largest high-risk cluster (cluster 4) of the present study was located. Hence, anemia has been proposed as a useful indicator of the burden and control of infectious diseases66.
Limited access to healthcare services may be another factor contributing to spatial clustering of anemia. The median travel time to health facilities located in rural areas is higher than in urban areas. A previous study showed that the largest travel time to the nearest healthcare facility was for travel from the north-east of the Amazon region and the southern areas of the highland and coastal regions, which are less urbanized67. These areas coincide with most of the high-risk clusters (cluster 1, cluster 5 and cluster 6) and highly prevalent areas (by Kriging interpolation) detected in the present study. Further studies should be conducted to evaluate the factors contributing to spatial clustering of anemia in Peruvian children.
All in all, a comprehensive approach must be implemented to reduce the gap in the prevalence of anemia between urban and rural areas. This should be directed to the factors determined in the present study to reduce this gap. Urbanization is constantly advancing; therefore, a reduction in the prevalence of anemia would be expected in upcoming years. In addition, healthcare providers are unequally distributed among urban and rural areas. Despite fewer people living in rural areas, inadequate allocation of resources may be present and could lead to inadequate healthcare attention68. Nonetheless, the COVID-19 pandemic could change the landscape, since strong restrictive measures were implemented in Peru to mitigate the effects of the pandemic, limiting access to health services.
This study should be interpreted considering its limitations. Given the cross-sectional design, causality cannot be determined. It would have been interesting to analyze other variables such as travel time to the nearest health center, dietary patterns, environmental exposures and parasitic infections, among others. Unfortunately, these variables were not available in the database. In addition, SaTScan detects circular shaped clusters, but not irregularly shaped clusters.
This study had several strengths. First, the DHS is nationally representative and has a large sample size. Moreover, data were weighted to correct sampling errors. The results are comparable with others from middle-income countries. Second, the measurement tool for anemia had an adequate performance. Third, this is the first study applying multivariate decomposition to identify factors contributing to the difference of anemia prevalence between urban and rural areas. Fourth, various spatial analysis techniques were employed, which allowed identification of hotspot areas, with high prevalence of anemia.
Conclusion
Despite efforts to reduce the prevalence of anemia, it remains high, with a large gap between urban and rural areas in Peru. Children´s compositional characteristics differences were significantly associated with the difference in anemia prevalence between rural and urban areas. Interventions targeting wealth index, mother´s education, employment and family planning would be helpful in reducing the gap of the anemia prevalence between residence areas. Anemia had a heterogeneous distribution at the inter- and intradepartmental level, with the presence of six high-prevalence clusters. Simultaneous adverse conditions jeopardize the health of children living in vulnerable areas as well as those in rural settings, hence multisectoral and intersectoral targeted actions are necessary to reduce these disparities. Peruvian policymakers should crack down on geographic disparities of anemia.
Acknowledgements
The authors thank engineer Jorge Cubas for his guidance on spatial analysis and Dr Daniel Powers for sharing with us the latest updates of the mvdcmp command.
References
Supplementary material is available on the live site https://www.rrh.org.au/journal/article/6936/#supplementary




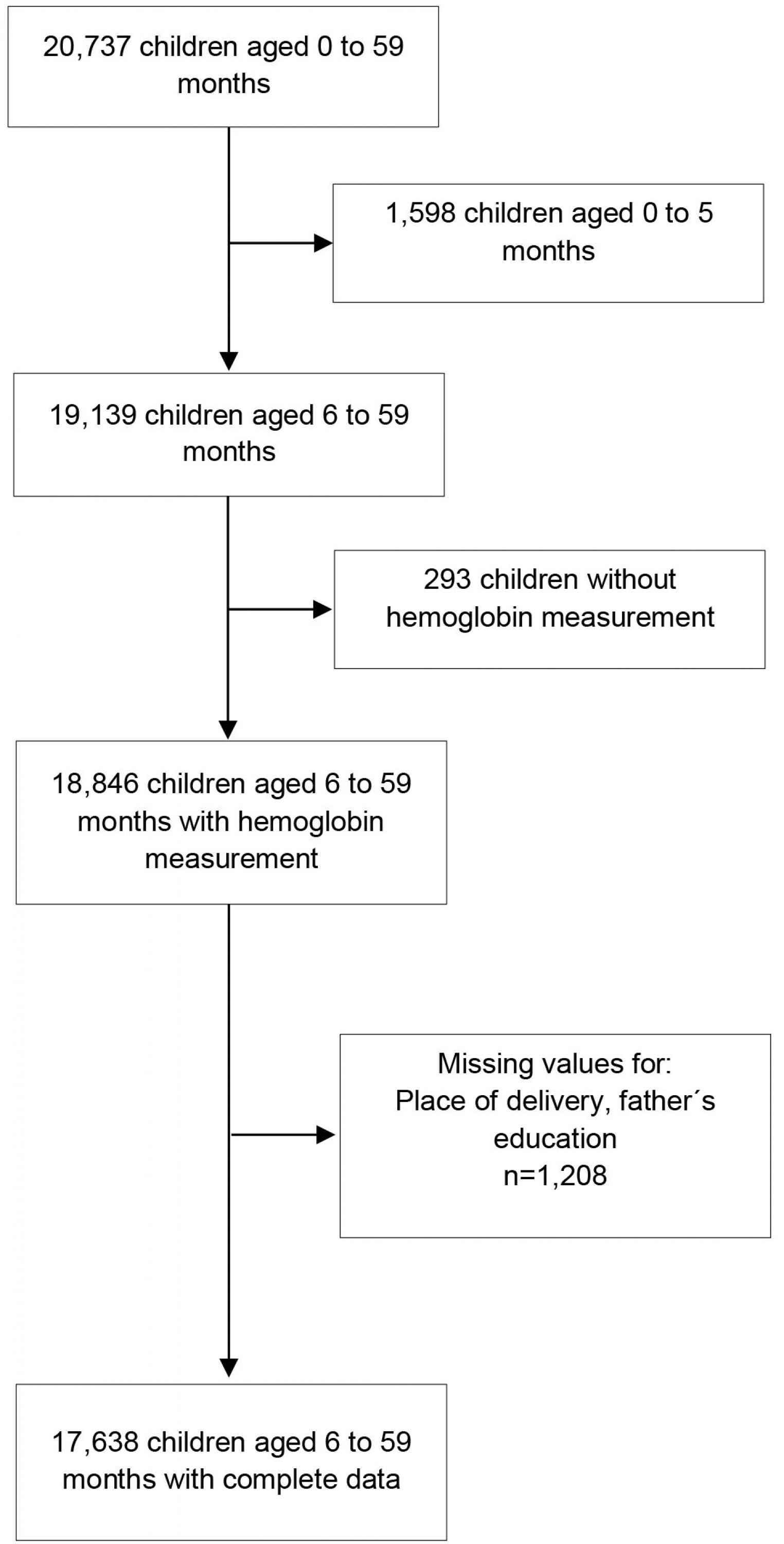 Supplementary Figure 1: Flowchart
Supplementary Figure 1: Flowchart