Introduction
In the USA, breast and colorectal cancer (CRC) are among the top three most common and deadly cancers in females1. It is estimated that breast and colorectal cancer resulted in 43 600 and 24 460 deaths among US females in 2021, accounting for nearly 24% and 13% of all female cancer deaths, respectively1. The 2020 Cancer Disparity Report by the American Association of Cancer Research (AACR) revealed that screening disparities continue to manifest between different racial groups, with many minority groups continuing to face poorer screening rates than their White counterparts2. Overall, breast cancer and CRC incidence have decreased over the past few decades and among females aged 50 years or above, in large part because of the implementation of screening and preventive care3. However, the pace of these improvements has not been equally distributed across racial groups and between urban and rural regions.
Between 1998 and 2005, rural residents were consistently lagging their urban counterparts in CRC screening, with ultimately 48% reported being up to date with CRC screening compared to 54% in urban counterparts by 20054. A 2012 Texas study found that rural residents were less likely to report CRC screening compared to those residents in a city center5. To better understand barriers to CRC screening in rural populations, a systematic review was conducted, encompassing studies between 1998 and 2017. It found specialty shortage, distance to a test facility and a lack of focus on preventative cancer screening to be some of the barriers to achieving parity with urban screening rates6. Similar trends were found in breast cancer screening. Using Nebraska cancer registry and insurance claims data, a retrospective analysis examining breast cancer screening between 2008 and 2012 found that urban areas had higher screening rates compared to rural populations and overall lower rates of late-stage breast cancer diagnoses7. The findings mirrored similar trends observed in earlier studies conducted in Missouri and Wisconsin8,9.
While numerous studies have investigated screening disparities between rural and urban groups, limited recent studies have sought to evaluate for a trend in screening disparities. Previous studies either used 1-year data or data pooled from several years to assess for screening differences and therefore are limited in their ability to assess for a trend5,7-9. Assessment for screening disparities over time is critical given the growing geographic disparity of healthcare resources in the USA. Rural hospital closures have been shown to affect rural residents’ access to health care. The US Government Accountability Office found that 64 rural hospitals closed between 2013 and 2017, which was double the amount in the preceding 5 years, and 3% of all rural hospitals10. In North Carolina, for example, this translated to 4.4 million rural residents residing in a county without an acute care hospital. Rural residents, particularly those elderly and poorer, reported skipping diagnostic imaging and preventative care due to local hospital closures11. Given the continued divergence in healthcare infrastructure between urban and rural regions, it is imperative to continue to investigate the trends in cancer screening between these disparate geographic populations.
In addition to geographic screening differences, racial/ethnic screening disparities have been an important research topic in the oncologic community. The 2020 AACR Cancer Disparities Progress Report revealed that African American women were more likely to be diagnosed with a more advanced stage breast cancer compared to White women, ultimately leading to an increased breast cancer death rate of 27.3 per 100 00 v 19.7 per 100 000, respectively2. Given screening rates were similar between African American and White women, the report suggested that African American women may be screened and followed at lower resourced facilities and that there may be an overestimation of mammogram utilization in this group. Racial/ethnic differences were also observed in colorectal cancer screening. In the 2020 AACR report, White Americans had an up-to-date screening rate of 63.7%, higher than that of African American, Hispanic, American Indian/Alaska Native and Asian groups, which had screening rates of 59.3%, 47.4%, 48.4% and 52.1%, respectively2. Prior studies suggest the lower screening rates among some groups, particularly American Indians and Alaska Natives, could be a result of worse access to resource-rich facilities that utilize endoscopic screening modalities3. Further studies describing screening differences between racial/ethnic groups are necessary to better understand changes in cancer screening patterns.
This study examined trends of breast cancer and CRC screening among females assigned at birth aged 50–74 years using the 2014–2019 Behavioral Risk Factor Surveillance System (BRFSS) data (except for 2017 due to lack of cancer-related data). A particular focus is placed on comparing urban and rural populations as well as screening differences by race/ethnicity. Hence, the aim is to provide the most up-to-date data in order to help guide public health officials and policymakers address the persistent disparity in cancer screening rates of this population.
Methods
This cross-sectional study analyzed nationally representative datasets from BRFSS 2014–2016, 2018–2019, the largest continuously conducted national health survey (eg health behaviors and healthcare use) in the non-institutional individuals residing in the USA. BRFSS samples are representative of each state and the nation, and the detailed information of study methods can be found elsewhere12. The survey was conducted by landline and cellphone with oversampling for underrepresented groups and adjustment for non-responders (the unadjusted and adjusted response rates varied by years; detailed information can be found elsewhere12). The response rates were 47.1% (cellphone) and 53.4% (landline) in 2019, 43.4% (cellphone) and 53.3% (landline) in 2018, and 46.3% (cellphone) and 47.7% (landline) in 2016, 47.2% (cellphone) and 48.2% (landline) in 2015, and 40.5% (cellphone) and 48.7% (landline) in 2014. Institutional review board approval was not required as the study utilized publicly available information.
Sample
An aggregated, age-adjusted prevalence measure was used to estimate the percentage of females assigned at birth (hereafter referred to as female) aged 50–74 years, non-institutional and living in the USA.
Measures
Major outcome variables: These were the proportion of up-to-date breast and CRC screening based on the US Preventive Services Task Force (USPSTF) recommendations. Up-to-date mammogram screening included individuals who had a mammogram screening in the previous 2 years13. Up-to-date colorectal cancer screening included individuals who had at least one of the following: a fecal occult blood test (FOBT) in the previous 24 months, flexible sigmoidoscopy in the previous 5 years or a colonoscopy in the previous 10 years14.
Baseline demographics: Baseline demographics were residential location (urban/rural areas; urban defined as residing within or immediately outside a city center), age (50–54, 55–59, 60–64, 65–69 or 70–74 years), race/ethnicity (non-Hispanic White; non-Hispanic Black; Hispanic/Latino; American Indian or Alaskan Native; Asian, Native Hawaiian or other Pacific Islanders; Other race), sexual orientation (heterosexual; lesbian; bisexual; something else), educational attainment (less than high school degree; high school degree or equivalent; some college and above), annual household income (less than US$25,000; US$25,000– US$49,999; US$50,000 or more), marital status (married; divorced/separated/widowed; single) and general health (good; fair; poor).
Healthcare access: Access comprised health insurance (yes/no), having a personal doctor or healthcare provider (only one; more than one; no), last checkup (never; within past year; within past 2 years; more than 2 years) and trouble with medication costs (yes/no).
Health behaviors: Behaviors included binge drinking (yes/no) and smoking status (current smoker; former smoker; never).
Statistical analysis
Population estimates of baseline demographics, healthcare access and health behaviors are presented by urban–rural residential areas (Table 1). An examination of the trends of breast cancer and CRC screening rates overall and by urban–rural residential area over time are presented and adjusted for confounders (Fig1). A bivariate analyses and logistic regressions of breast cancer and CRC screening in relation to urban–rural residential area and race/ethnicity adjusted for confounders were performed (Table 3). Odds ratios (OR) with 95% confidence intervals (CI) were reported, and two-tailed p-values less than 0.05 were considered statistically significant. All significant tests were adjusted for the BRFSS’s complex sample design using the Centers for Disease Control and Prevention formula. The analysis was conducted using Stata v17.0 (StataCorp LP; http://www.stata.com).
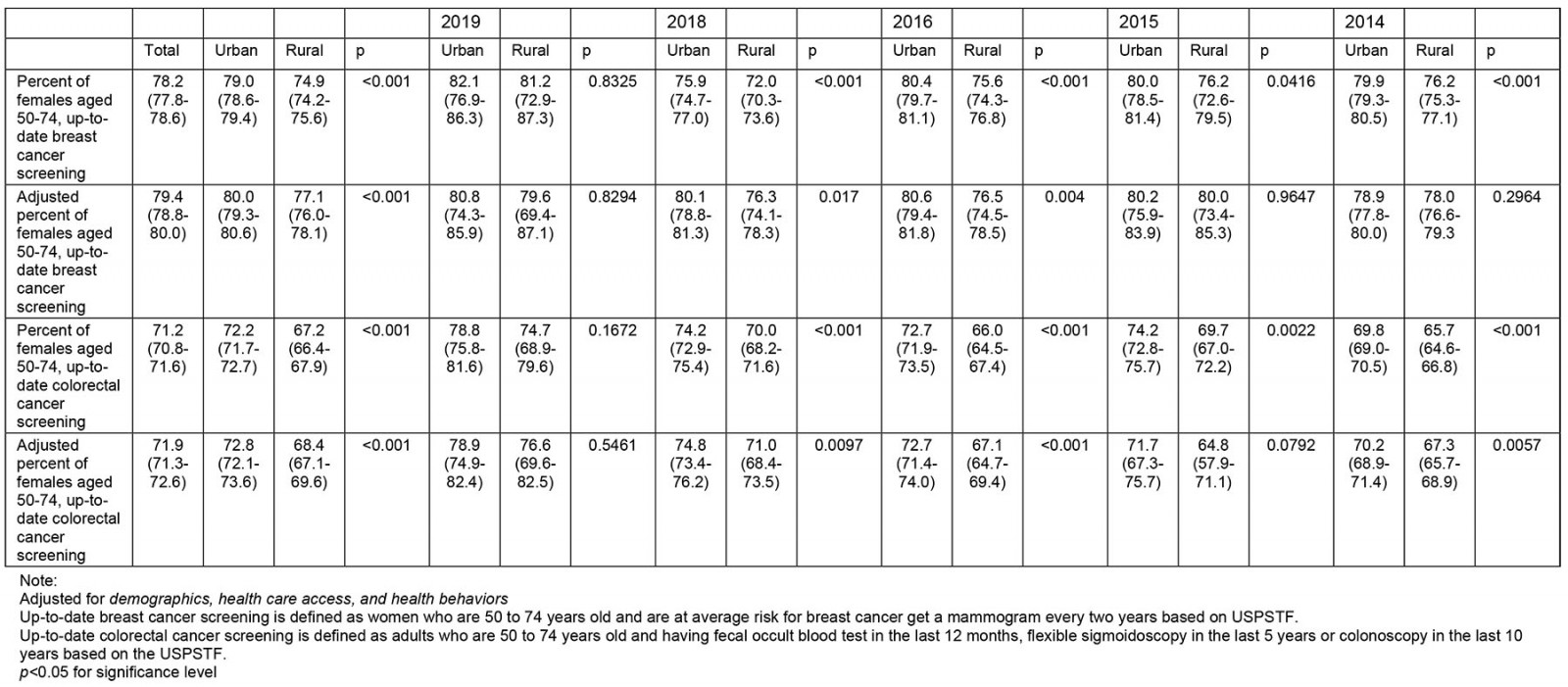
Table 1: Weighted characteristics of US urban and rural females aged 50–74 years, Behavioral Risk Factor Surveillance System 2014–2019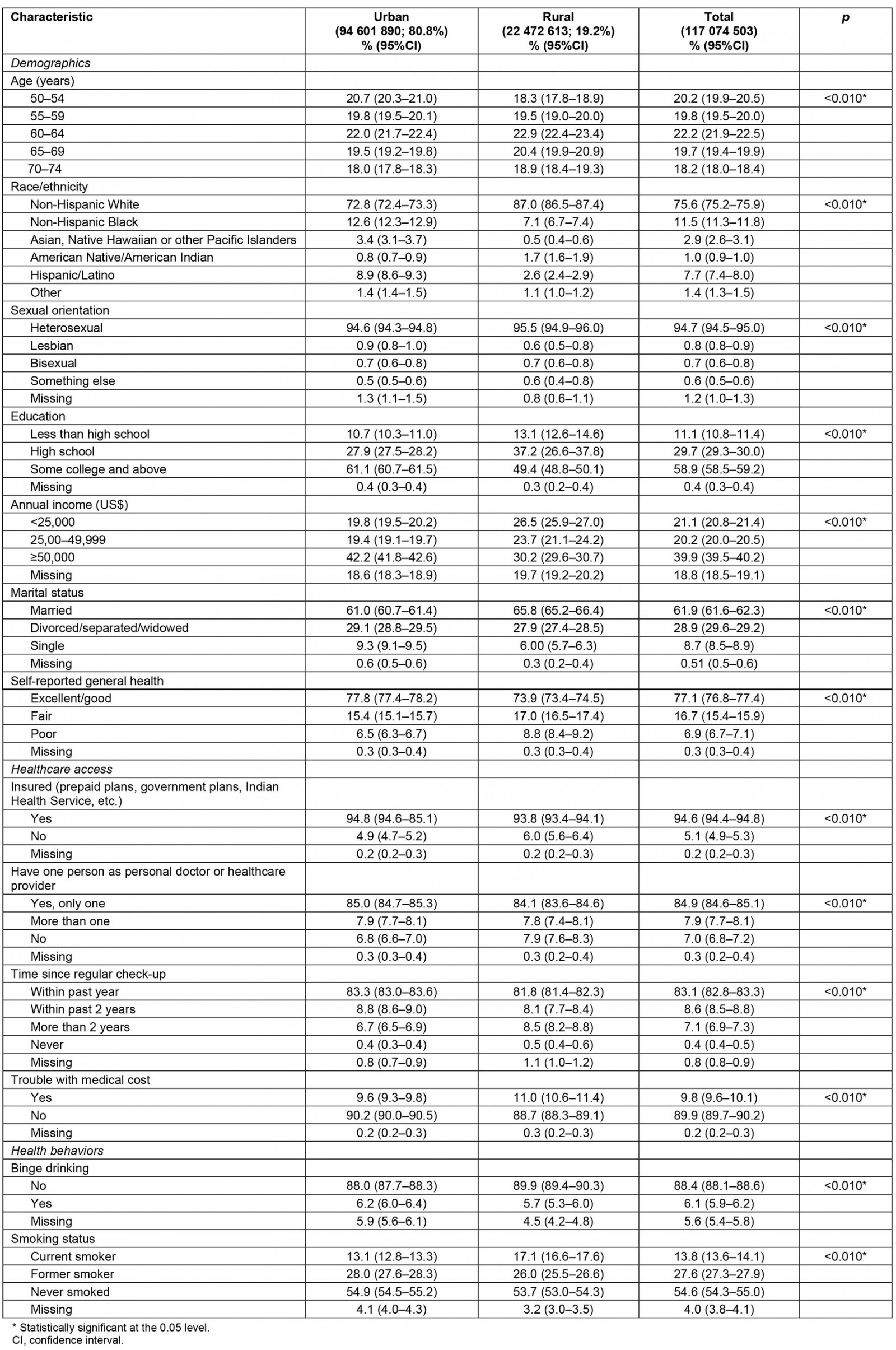
Table 2: Weighted, adjusted logistic regression† of up-to-date breast cancer¶ and colorectal cancer§ screening in relation to US urban–rural area and race/ethnicity among females aged 50–74 years, Behavioral Risk Factor Surveillance System 2014–2019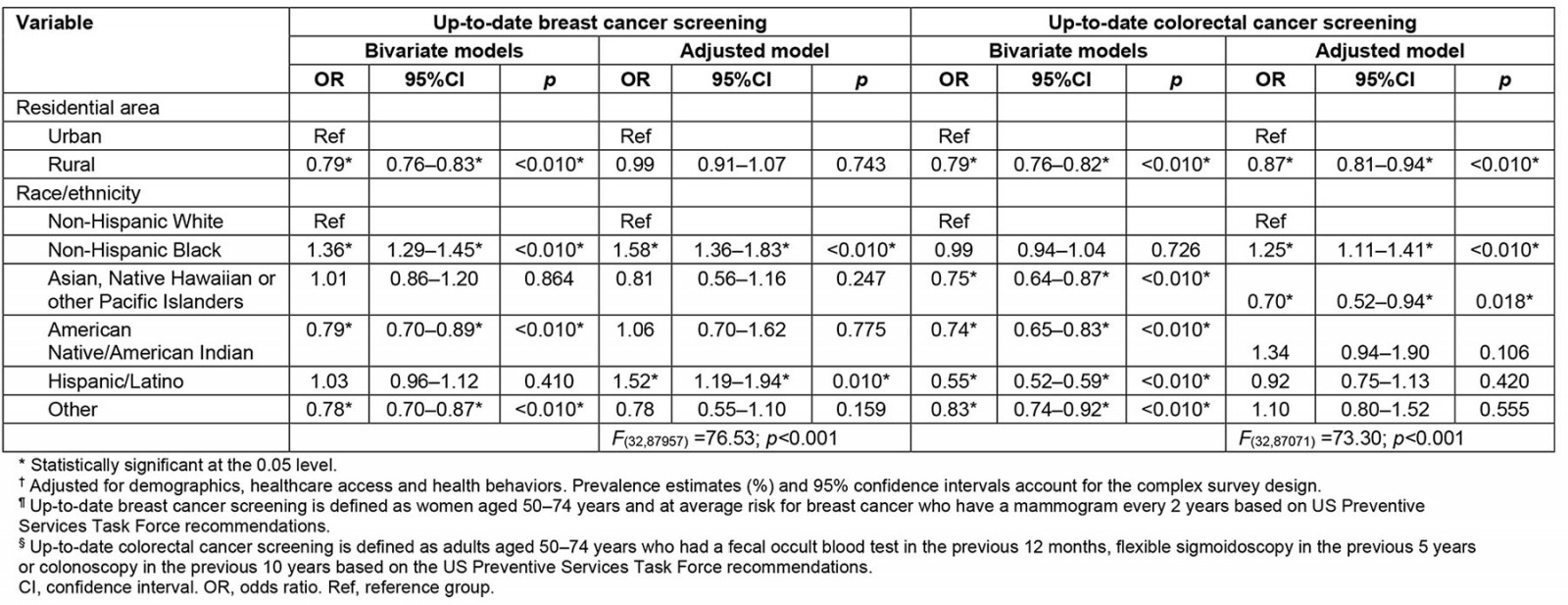
Table 3: Weighted logistic regression of colonoscopy screening and correlates in females assigned at birth aged 50–74 years, US rural populations, Behavioral Risk Factor Surveillance System 2014–2019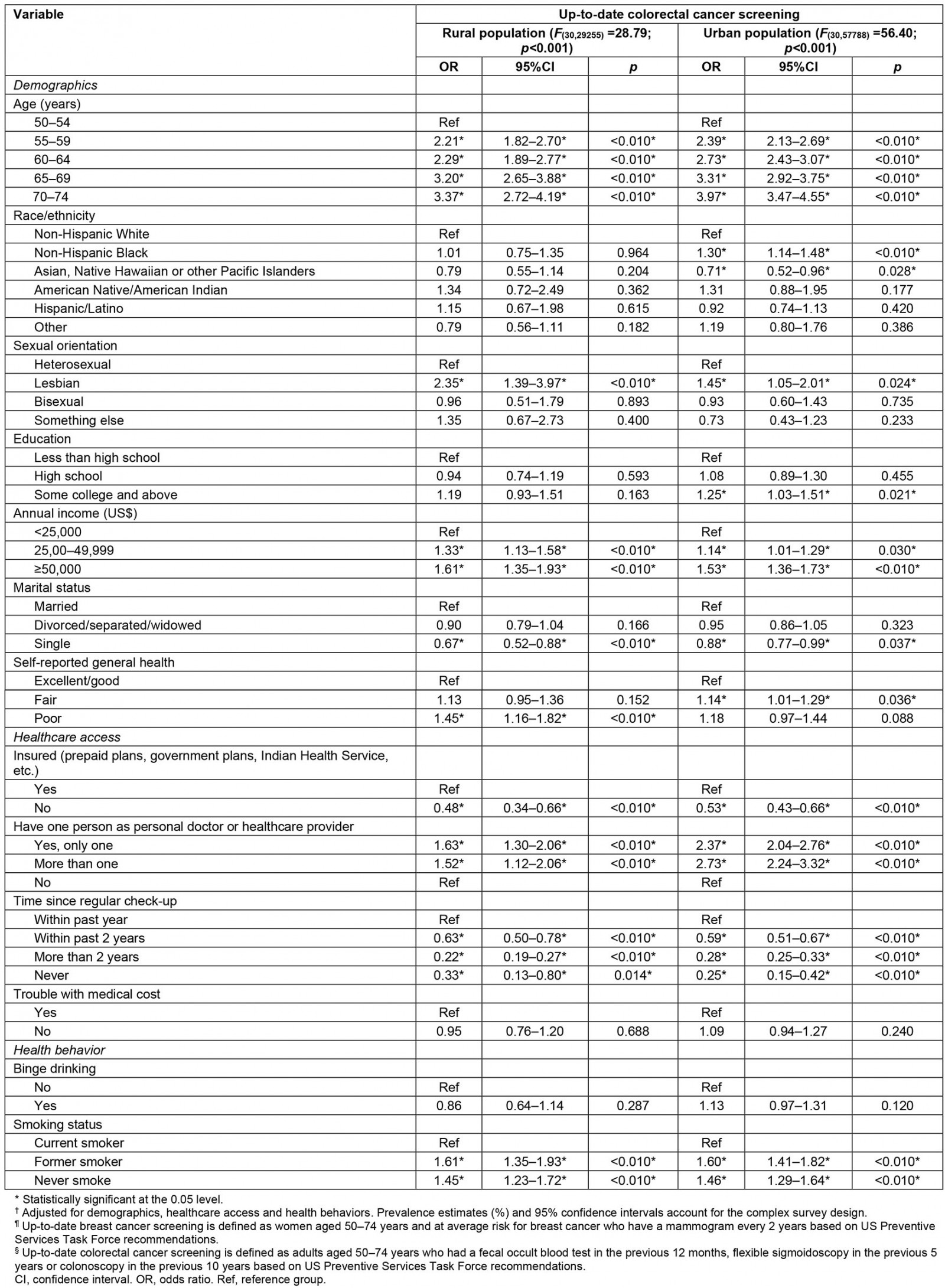
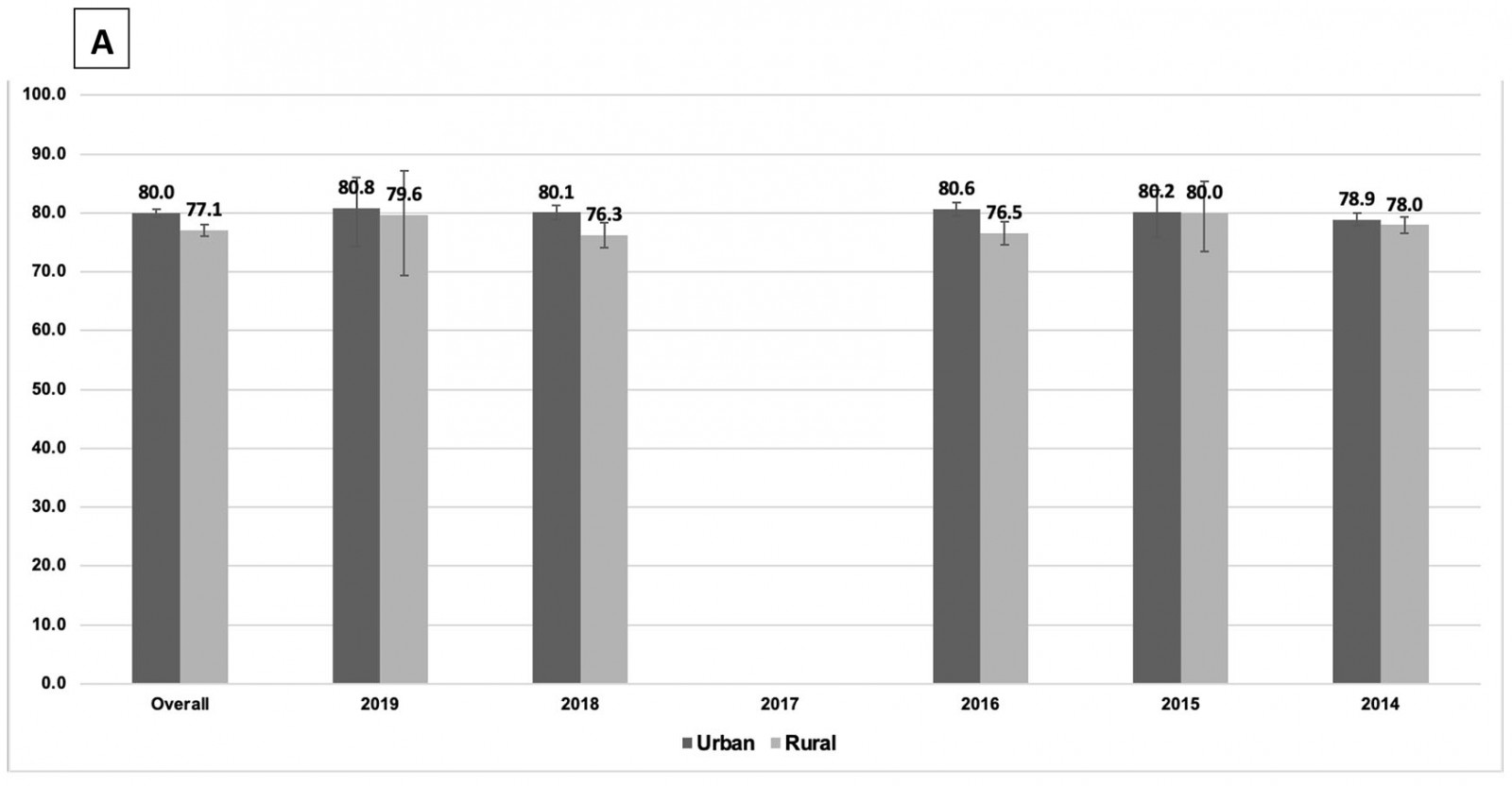
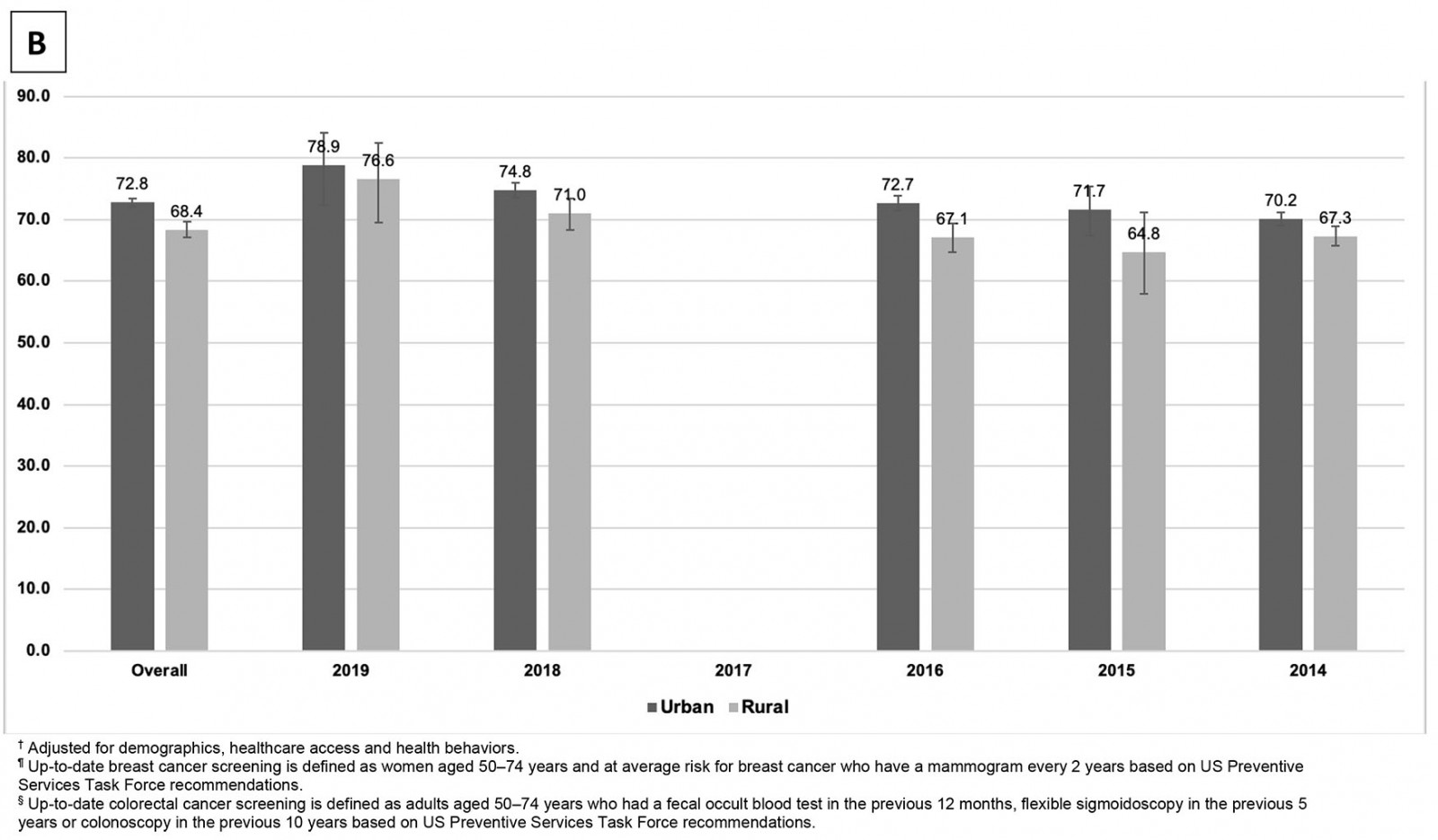 Figure 1: Weighted, adjusted† prevalence of up-to-date breast cancer¶ (A) and colorectal cancer§ (B) screening in females aged 50–74 years by US urban–rural area, Behavioral Risk Factor Surveillance System 2014–2019.
Figure 1: Weighted, adjusted† prevalence of up-to-date breast cancer¶ (A) and colorectal cancer§ (B) screening in females aged 50–74 years by US urban–rural area, Behavioral Risk Factor Surveillance System 2014–2019.
Ethics approval
The data set does not involve human subjects (as defined by federal regulations and guidance) and therefore requires neither institutional review board review nor an exempt determination.
Results
As shown in Table 1, the estimated urban population was 94 601 890 (80.8%; 95%CI=80.6–81.0), and 22 472 613 (19.2%; 95%CI=19.0–19.4) for the rural population. There were significant differences in all demographics, healthcare access and health behaviors by urban–rural residential area. Rural populations have significantly lower proportions of racial and sexual minority residents, lower education and income levels, and a higher proportion of self-identified fair and poor health compared with their urban peers. Moreover, a higher proportion of rural residents reported being married as compared with urban residents. The rural residents had significantly lower rates of health insurance coverage and regular check-ups compared to their urban peers. They reported higher rates of medical cost burden, binge drinking and current smoking.
Figure 1 shows temporal trends in screening disparities, the adjusted prevalence of up-to-date breast cancer and CRC screening for both urban and rural populations. The weighted, adjusted and unadjusted prevalences of up-to-date breast cancer and CRC screening between 2014 and 2019 (excluded 2017) are shown in Supplementary table 1. Overall, urban areas had higher rates of breast cancer screening (79.0%; 95%CI=78.6–79.4) compared to rural areas (74.9%; 95%CI=74.2–75.6; p<0.001); rates of CRC screening were 72.2% (95%CI=71.7–72.7) for urban areas and 67.19% (95%CI=66.4–67.9; p<0.001) for rural areas. After adjusting for demographics, healthcare access and health behaviors, the adjusted prevalence of breast cancer screening in the urban population was 80.0% (95%CI=79.3–80.6) v 77.1% in the rural group (95%CI=76.0–78.1; p<0.001). Adjusted prevalence of CRC screening in the urban population was 72.8% (95%CI=72.1–73.6) compared to 68.4% in the rural group (95%CI=67.1–69.6; p<0.001).
The gaps of breast cancer and CRC screening rates between urban and rural populations vacillated between 2014 and 2019 after adjusting for confounders. The breast cancer screening rates were significantly greater in urban populations compared to their rural counterparts in 2016 (80.6% (95%CI=79.4–81.8) v 76.5% (95%CI=74.5–78.5); p<0.001) and 2018 (80.1% (95%CI=78.8–81.3) v 76.3% (95%CI=74.1–78.3); p=0.017), but not in 2014, 2015 and 2019. Likewise, the CRC screening rates were significantly greater in urban populations compared to their rural counterparts in 2014 (70.2% (95%CI=68.9–71.4) v 67.3% (95%CI=65.7–68.9); p<0.010), 2016 (72.7% (95%CI=71.4–74.0) v 67.1% (95%CI=64.7–69.4); p<0.010) and 2018 (74.8% (95%CI=73.4–76.2) v 71.0% (95%CI=68.4–73.5); p=0.097), but not in 2015 and 2019.
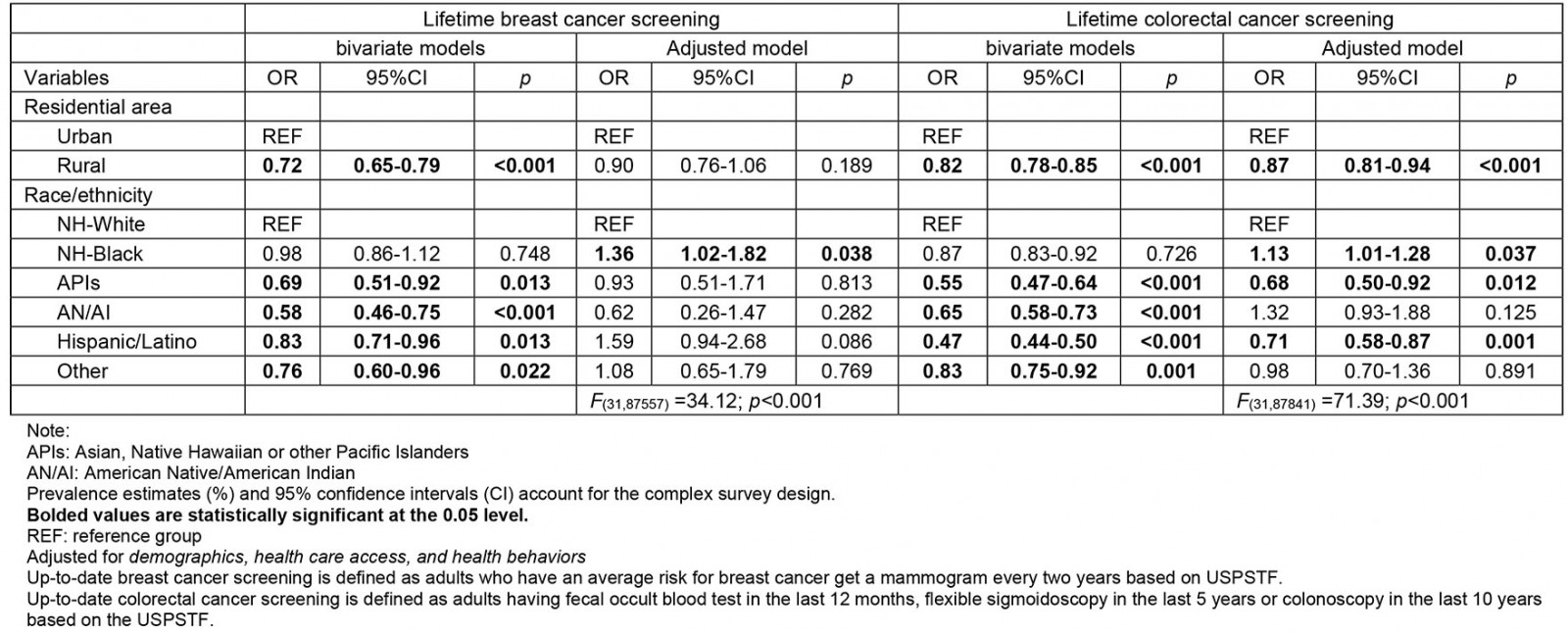
Furthermore, it was found that receiving an up-to-date breast cancer screening was associated with race/ethnicity, but not with urban–rural residential areas after adjusting for all covariates (demographics, healthcare access and health behaviors; Table 2). Non-Hispanic Black (OR=1.58; 95%CI=1.36–1.83) and Hispanic (OR=1.52; 95%CI=1.19–1.94) women were significantly more likely to receive an up-to-date breast cancer screening than their non-Hispanic White peers.
Receiving up-to-date CRC screening was associated with urban–rural residential area and race/ethnicity after adjusting for confounders (Table 3). Rural women were less likely to have up-to-date CRC screening than those in urban areas (OR=0.87; 95%CI=0.81–0.94). Non-Hispanic Black women were significantly more likely to have up-to-date CRC screening (OR=1.25; 95%CI=1.11–1.41), whereas Asian, Native Hawaiian or other Pacific Islanders were less likely to have up-to-date CRC screening (OR=0.70; 95%CI=0.52–0.94) than their non-Hispanic White peers.
Furthermore, multivariable models of an up-to-date CRC screening stratified by urban–rural residential area revealed significant differences in demographic, healthcare access and behavioral factors (Table 4). Among urban women, those who were non-Hispanic Black (OR=1.30; 95%CI=1.14–1.48), had some college education or above (OR=1.25; 95%CI=1.03–1.51), reporting fair general health (OR=1.14; 95%CI=1.01–1.29), being lesbian in sexual orientation (OR=1.45; 95%CI=1.05–2.01) were more likely to have up-to-date CRC screening. Asian, Native Hawaiian or other Pacific Islanders were less likely to receive CRC screening (OR=0.71; 95%CI=0.52–0.96) compared to their non-Hispanic White peers.
For rural women, race/ethnicity and education were not significant factors for an up-to-date CRC screening. Those reporting poor general health (OR=1.45; 95%CI=1.16–1.82) or being lesbian in sexual orientation (OR=2.35; 95%CI=1.39–3.97) were more likely to have up-to-date CRC screening.
Discussion
This study sought to further explore the temporal trend in breast cancer and CRC screening among women aged 50–74 years between urban and rural populations, and to better define the disparities in screening. When examining the rural and urban population as a whole between 2014 and 2019, there was a significantly higher rate of breast cancer and CRC screening in urban compared to rural populations after adjusting for confounders. When looking at annual prevalence, breast cancer screening in urban areas compared to rural populations was significantly higher in 2016 and 2018, but appeared to be similar in 2019. A similar trend was observed in CRC screening. Screening differences for both cancers were observed in different racial groups.
Urban women had a significantly higher prevalence of breast cancer screening compared to rural women between 2014 and 2019. This finding is consistent with previous findings in mammography in urban versus rural populations15-17. Zhang et al examined data for women aged 50–69 years from the 1994 US National Health Interview Survey, and found that the prevalence of mammograms in urban women was statistically different at 68% compared to 61% in rural women (p<0.05)15. Using the BRFSS data between 1994 and 2004 for women aged 40 years and older, Doescher and Jackson found that while the rate of mammography screening had been increasing in all regions, significant differences remained between urban and rural women (75% v 70%), which was corroborated by a more recent study by Tran and Tran using the 2012–2016 BRFSS data16,17.
Various barriers to cancer screenings exist in rural populations. Distance to mammography centers may play a role, as demonstrated by Chandak et al using a spatial cluster analysis, which found some rural areas had longer distances to mammography centers and had higher rates of late-stage cancer diagnosis7. Longer drive times seen in rural areas have been associated with decreased breast cancer screening frequency9. Lower breast cancer screening uptake has been associated with lower levels of education and lack of insurance in rural populations, although in this study no differences were seen in regard to education18.
Consistent with previous findings, non-Hispanic Black and Hispanic women had increased odds of receiving breast cancer screening compared to their non-Hispanic White counterparts17. To better understand these racial/ethnic differences, further analysis was performed, looking at lifetime screening data (Supplementary table 2). Non-Hispanic Black women remained significantly more likely to receive lifetime breast cancer screening (OR=1.36; 95%CI=1.02–1.82; p=0.038) but not in Hispanics compared to their non-Hispanic White counterparts (OR=1.59; 95%CI=0.94–2.68; p=0.086). While selection bias could potentially account for this discrepancy, it could be postulated that while Hispanics overall have less breast cancer screening compared to non-Hispanic White women, recent public health interventions could be resulting in higher proportions of Hispanic individuals with more recent cancer screening compared to non-Hispanic Whites19,20. When examining temporal trends of breast cancer screening, the difference in breast cancer screening rate between rural and urban areas became smaller and was not statistically significantly different in 2019. The narrowing in screening differences was consistent with the trends found in previous studies15-17. While screening differences in 2019 were not statistically significant, they remain clinically significant as small variances at the population level equate to thousands of women without up-to-date breast cancer screening. Higher insurance coverage during this period could explain the improvement in screening20. This is evidenced by a previous study using BRFSS data that found increased cancer screening rates among lower income individuals in states with expansion Medicaid, the publicly funded health insurance program for low-income individuals21. Medicaid expansion has particularly helped rural populations, with an 8.5% increase in insurance coverage compared to 4.1% in the urban population between 2011 and 201522. As Medicaid expansion becomes law in more states, further studies will be needed to evaluate its impact on cancer screening.
Results of the present study indicated that, similar to breast cancer screening rate, the CRC screening rate was overall higher in urban women compared to rural women. This is in line with previous studies23. Using the 1998–2005 BRFSS data, Cole et al found that, among individuals aged more than 50 years, CRC screening prevalence was 54.0% in urban individuals compared to 48.1% in rural individuals after adjusting for confounders4. In addition, their study showed a consistent increase in CRC screening in both rural and urban populations. Using the 2012 BRFSS data, Ojinnaka et al found that rural individuals had lower odds of ever having CRC screening compared to urban individuals5. A systematic review found the barriers to CRC screening in rural USA include lack of perceived privacy, long distances to specialist and testing facilities, and lack of focus on cancer prevention6. While urban women had an overall higher prevalence of screening during this study period, by 2019 there was no significant CRC screening difference. As already discussed, the expansion of health insurance coverage in rural populations has been associated with increased cancer screening uptake and could account for improved screening rates. In addition, reduction in out-of-pocket costs for colonoscopy after the Affordable Care Act was implemented has been associated with increased colonoscopy rates in rural populations23.
Disparities of cancer screening existed within the different races and ethnicities. Urban non-Hispanic Blacks had increased rates of CRC screening compared to non-Hispanic Whites, which was consistent with prior studies24,25. Cook et al analyzed electronic health records collected from ten community health centers in eight states and revealed non-Hispanic Blacks and Hispanics were more likely to receive pap tests compared to their non-Hispanic White counterparts after adjusting for demographic variables25. Likewise, Holden et al found that, among those who were uninsured, both non-Hispanic Blacks and Hispanics were more likely to have pap tests and mammograms compared to non-Hispanic White women26. One possible explanation for differences in cancer screening uptake is the effectiveness of public health interventions. Wells et al performed a systematic review of community health worker–led screening interventions, which appeared to be effective in improving screening rates in urban settings and also by racial/ethnic similarity with the community health worker27. The greater density of racial/ethnic minorities in urban areas that also have a greater concentration of healthcare services, coupled with effective interventions, provides a plausible explanation for uninsured Hispanic and non-Hispanic Black individuals having higher odds of preventive service receipt than non-Hispanic White peers25.
Urban Asian, Native Hawaiian or other Pacific Islander women had decreased odds of CRC screening compared to non-Hispanic White women. The decreased rate in CRC screening has previously been observed in Asian groups, which has been suggested to be due to health literacy barriers, decreased emphasis of cancer prevention, culture-related cancer stigma, and fear of finding a cancer diagnosis and burden on their family28. There was no observed difference in the odds of CRC between rural ethnicities. This could be due to a less diverse rural population in this sample and therefore less likely to observe significant differences. Rural women with self-reported poor health and urban women with self-reported fair health had increased odds of obtaining CRC screening than those who reported excellent/good health. This may be due to sicker individuals having increased interface with the healthcare system and, therefore, more regular medical check-ups. More recent follow-up, presence of a personal physician and having insurance were all observed to be positive predictors for increased CRC screening rate in both rural and urban groups, which was consistent with the previous literature23.
Limitations
BRFSS data are self-reported, resulting in some misclassification of age, race, education, and income17. Reliance on self-reporting behaviors such as alcohol use and smoking status makes this study prone to recall bias. Individuals reporting breast cancer and CRC screening may have had indications for screening outside of USPSTF guidelines, such as based on risk behaviors, symptoms and family history. Therefore, the outcomes could be subject to misclassification; however, it is likely to be presented in both urban and rural groups equally27. The focus on women limits the generalizability of this study. Finally, no BRFSS data were collected in 2017 regarding this topic, and therefore a full assessment cannot be made during the study period.
Conclusion
This study examined whether disparities existed between urban and rural women aged 50–74 years for breast cancer or CRC screening and how those disparities manifested by race. The findings show that disparities between urban and rural women for up-to-date breast and CRC screening exist across the study period. Although the screening differences appear to be narrowed by 2019, they remain clinically significant. Barriers to screening have included distance to screening centers, lack of insurance and perceived privacy, poor health literacy and decreased focus on cancer screening. As breast cancer and CRC remain the major contributors to annual cancer deaths in the USA, the implementation of screening initiatives targeting under screened rural regions and racial groups can contribute to reducing the cancer-caused mortality in women.
References
Supplementary material is available on the live site https://www.rrh.org.au/journal/article/7339/#supplementary