Introduction
Geographical location is increasingly recognised as a contributor to health inequity. Compared to living in metropolitan areas, living rurally is associated with a higher rate of disease and worse health outcomes worldwide1. Past research shows that there is a relationship between rurality and illness, with those living in rural areas having higher rates of chronic illness, including congestive cardiac failure2, heart disease, diabetes3, kidney disease, stroke, type 2 diabetes and depression4.
One underexplored chronic disease within rural areas is obstructive sleep apnoea (OSA). OSA is a condition where a person repeatedly pauses in their breathing while asleep because their airways become wholly or partially blocked. These pauses in breathing cause significant and problematic decreases in oxygen levels5. The prevalence of OSA among the general population is 7–30%, depending on the severity classification utilised, and is higher in men and older people6-8. Untreated OSA stresses the body, causing increased blood pressure, heart rate and heart rate variability9,10. OSA is strongly associated with serious comorbidities, including increased risk of hypertension11,12, heart attack, stroke, diabetes13,14, major depression15,16 and early mortality17,18. These comorbidities highlight the importance of early diagnoses and treatment of OSA.
Like many other countries, Australia has limited access to health services and support in regional and rural areas, including a systemic shortage of GPs and specialists19-21. This inequity is a potential contributor to the higher levels of OSA diagnosis and severity of disease seen in rural men, alongside associated comorbidities19,22. For example, an overnight sleep laboratory polysomnography is the gold standard of OSA diagnosis. However, rural patients have access barriers, including higher costs, and long wait times and travel distances, as these services are more likely to be available in metropolitan locations. These barriers can result in delayed diagnosis.
Further, the lack of appropriately skilled OSA technicians and sleep specialists in rural areas may lead to a reduction in or discontinuation of continuous positive airways pressure (CPAP) device usage. To date, no study has explored the potential effects of these barriers on OSA diagnosis by comparing rural and metropolitan men. This study compares the prevalence of OSA risk and diagnosis in metropolitan, regional and rural populations. We hypothesise that men living in rural and regional areas would have higher rates of OSA risk but lower diagnoses of OSA.
Methods
Study design and setting
Cross-sectional secondary analysis was performed on wave 2 of the Ten to Men study, an Australian longitudinal study of male health23. The study was commissioned by the Commonwealth Department of Health in 2011 in response to the 2010 National Male Health Policy, and is Australia’s first national longitudinal study focusing exclusively on male health and wellbeing.
Procedure
The cohort was recruited using a random, stratified, multi-stage and cluster sampling method, designed to select males aged 10–55 years24. Wave 2 data collection was commenced between November 2015 and May 2016 by the University of Melbourne, with Roy Morgan Research undertaking the data collection and initial data processing for waves 1 and 2. For the Ten to Men study, the wave 2 sample size was 11 936 and for the wave 3 study it was 7523. The random selection of participants, with a response rate of 35%23, ensured a broad range of backgrounds and life experiences. For the present study, only participants aged over 18 years were included in the analysis. Wave 1 data were excluded because they were collected more than 10 years ago (October 2013 and July 2014) and deemed out of date for analysis. Data for a total of 10 513 men were included from wave 2 and 7262 from wave 3.
Measurements
Location: Rural, regional and metropolitan location was determined by the Modified Monash Model (MMM) Classification code. The model measures remoteness from 1 to 7 (1. metropolitan, 2. regional centre, 3. large rural, 4. medium rural, 5. small rural, 6. remote, 7. very remote). The MMM was developed by the Australian Government Department of Health to categorise metropolitan, regional, rural and remote areas according to geographical remoteness and town size, as defined by the Australian Bureau of Statistics (ABS)25. While regional centres do not have the same high provision of healthcare services as metropolitan cities, they generally have better healthcare access than rural areas. Therefore, this study consolidated the scale into three categories: metropolitan cities (1), regional centres (2) and rural areas (3–7). Participants who did not report a location or answer ‘yes’ or ‘no’ to being treated for OSA were excluded from the analysis.
Socioeconomic status: Socioeconomic status was measured using the Socioeconomic Indexes for Areas (SEIFA)26. The SEIFA ranks Australian locations (postcode or similar area) according to residents’ access to material and social resources and their ability to participate in society, relative to what is commonly experienced or accepted by the wider community. The SEIFA ranking is derived from a combination of income, education, employment, housing and other variables from ABS census data and postcode26.
OSA risk: OSA risk was measured using the STOP-Bang, a validated tool for screening for OSA risk27. To score at intermediate/high risk, a person had to score ‘yes’ to three or more of these questions: ‘Do you snore loudly, feel tired/fatigued, stop breathing/gasp during sleep, have high blood pressure, are you aged over 50 years, male gender, BMI >35 kg/m2, neck circumference 16 inches (~40.5 cm) or more. As neck circumference was not collected as part of the Ten to Men study, a score of ‘no=0’ was given as per STOP-Bang instructions27. The STOP-Bang scores were grouped into a binary variable: 1=low risk of OSA, 2=intermediate/high risk of OSA.
OSA diagnosis/treatment received: This was determined using answers to the following questions in the data: ‘Have you been treated for any of these conditions in the past 12 months? Sleep apnoea (yes/no)’, and ‘Have you been treated for any of these conditions in your lifetime? Sleep apnoea (yes/no)’.
Ethics approval
Original ethics approval for The Australian Longitudinal Study on Male Health was approved by the University of Melbourne Human Research Ethics Committee (HREC 1237897 and 1237376). Participants provided written consent for their participation. In addition, ethics approval was sought by La Trobe HREC to conduct the secondary analysis (HREC 21346).
Statistical analysis
One-way analysis of variance (ANOVA) and Bonferroni post-hoc tests were performed to compare characteristics between the three location groups (metropolitan, regional, rural). The χ2 analysis was used to compare OSA symptoms, risk and diagnosis between groups. A value of p<0.05 was considered statistically significant. Odds ratios (OR) and 95% confidence intervals (95%CI) were calculated based on expected and observed numbers. Further regression analysis was completed, controlling for known factors of OSA diagnosis, including age, BMI, SEIFA and combined household income. All analyses were conducted using IBM SPSS, v27 (IBM; https://www.ibm.com/products/spss-statistics).
Results
Data for 10 513 men were analysed in wave 2 and 7262 in wave 3. The characteristics of waves 2 and 3 are outlined in Table 1.
Table 1: Characteristics of participants in samples from waves 2 (n=10 513) and 3 (n=7262) of Ten to Men study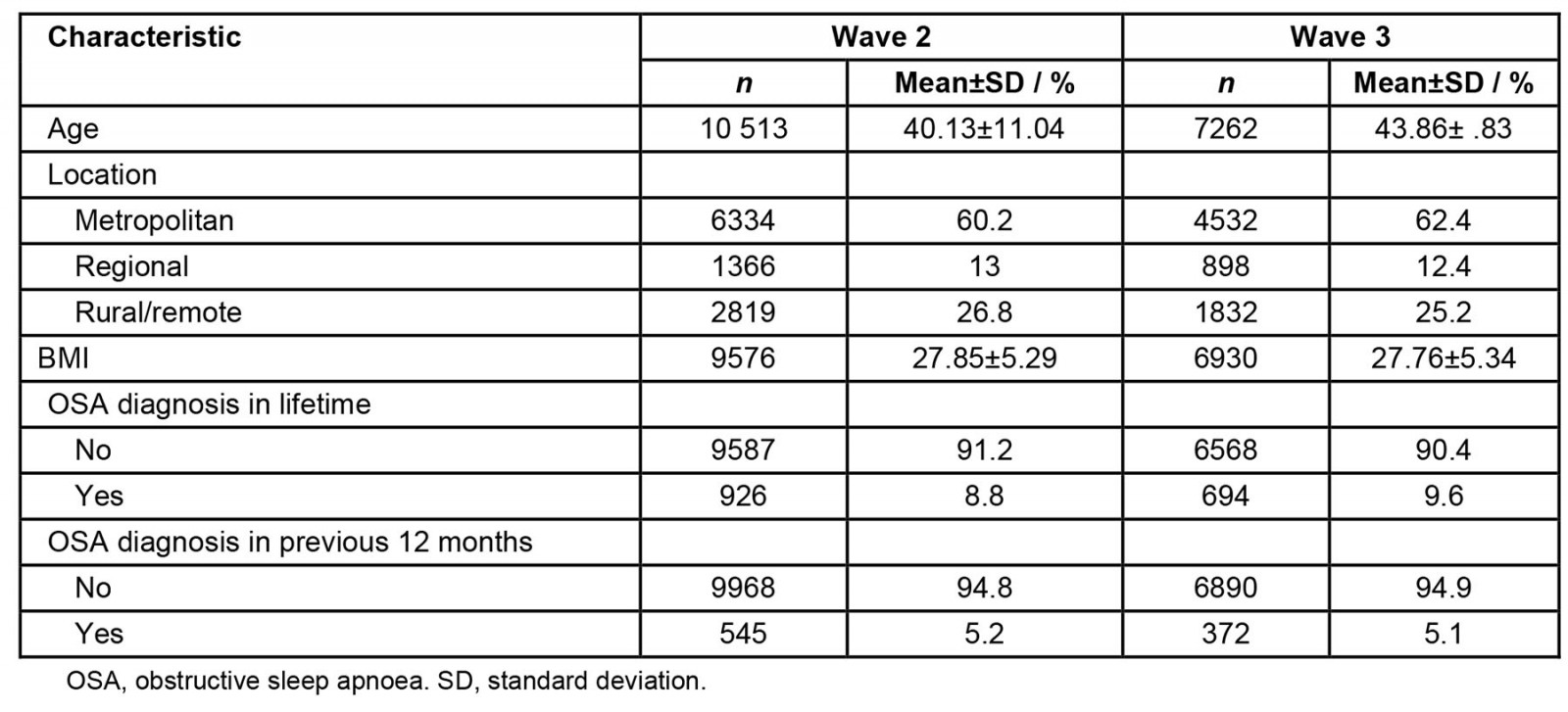
Wave 2 OSA
Differences in OSA risk: Results from the χ2 analysis showed that there was a significant association between where men lived and whether they were experiencing snoring (χ2(1)=22.51, p<0.001), difficulty breathing while sleeping (χ2(1)=15.49, p≤0.001) or high blood pressure (χ 2(1)=13.12, p=0.001). No differences were found in fatigue (p=0.392). There was a significant association between whether an individual lived rurally and whether they were deemed at risk of OSA (χ2(1)=29.61, p<0.001), with a higher proportion of men living rurally being at risk of OSA (38.7%, p<0.0001) compared to metropolitan men (32.5%). There were no associations for those living in regional areas compared with either metropolitan or rural areas.
Odds ratio analysis found that those living in rural areas were 1.30 times more likely to have high blood pressure (95%CI 1.13–1.48, p=0.002), 1.22 times more likely to report snoring (95%CI 1.12–1.34, p<0.001) and 1.27 times more likely to report difficulty breathing (95%CI 1.123–1.44, p<0.001) than those living in a metropolitan area. After removing participants who reported being diagnosed with OSA in their lifetime, 9094 men were included in the STOP-Bang analysis. There was, once again, a significant association between whether an individual lived rurally and whether they were at risk of OSA (χ2(1)=28.51, p<0.001), with a higher proportion of men living in rural areas being at risk of OSA (37.7%, p<0.0001) compared to metropolitan areas (32.6%). Odds ratio analysis showed that those in rural areas were 1.47 times (95%CI 1.22–1.78) more likely to be classified as high risk for OSA, according to the STOP-Bang analysis, than those in metropolitan areas. There was no association between those living in regional areas, compared with either metropolitan or rural areas.
OSA diagnosis/treatment received: The rate of men who had received OSA treatment during their lifetimes was associated with location (χ2(1)=10.361, p=0.006) and being diagnosed in the previous 12 months (χ2(1)=16.331, p<0.001). Men living in rural areas were 1.26 times more likely to have received treatment for OSA during their lifetimes (95%CI 1.09–1.47, p=0.002) and 1.47 times more likely to have been diagnosed with OSA during the previous 12 months (95%CI 1.22–1.78, p<0.001) than men in metropolitan areas. There was no association between those living in regional areas, compared with either metropolitan or rural areas.
Comparisons between metropolitan, regional and rural characteristics: There was a significant difference in age, BMI and SEIFA between the different locations (Table 2). Results from the regression analysis showed that age, BMI, SEIFA and combined household income remained significant factors for OSA diagnosis and treatment received in a lifetime but not rural location These factors contributed to 13.3% of the variance (Nagelkerke R2=0.133; χ 2 (3)=590.90, p<0.001) in the model (Table 3).
Table 2: Metropolitan, regional and rural comparisons for sample (n=10 513) from wave 2 of Ten to Men study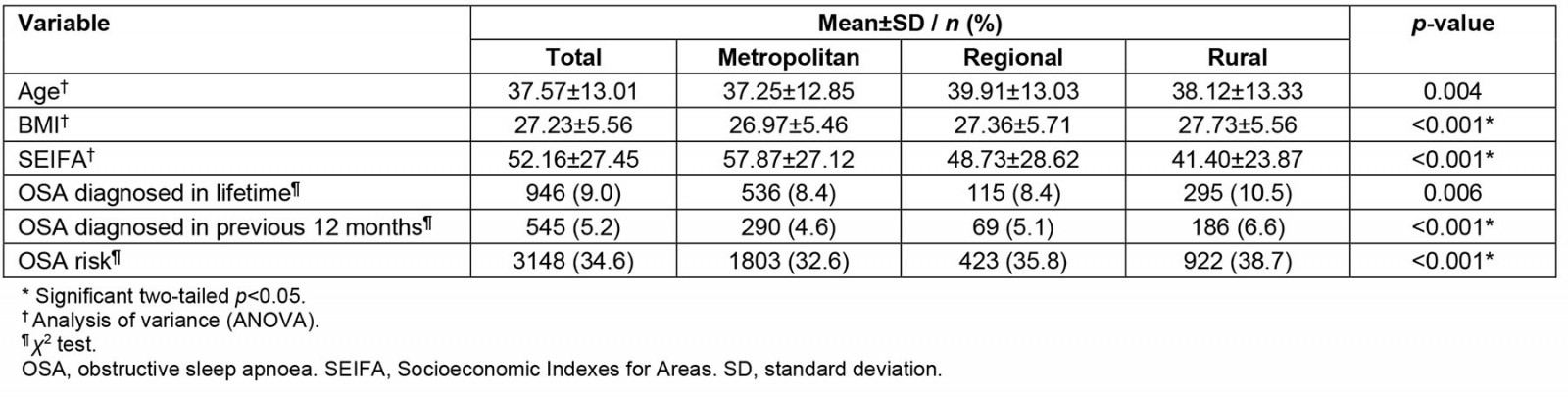
Table 3: Regression analysis exploring factors for OSA diagnosis in a lifetime (yes/no) for sample (n=10 513) from wave 2 of Ten to Men study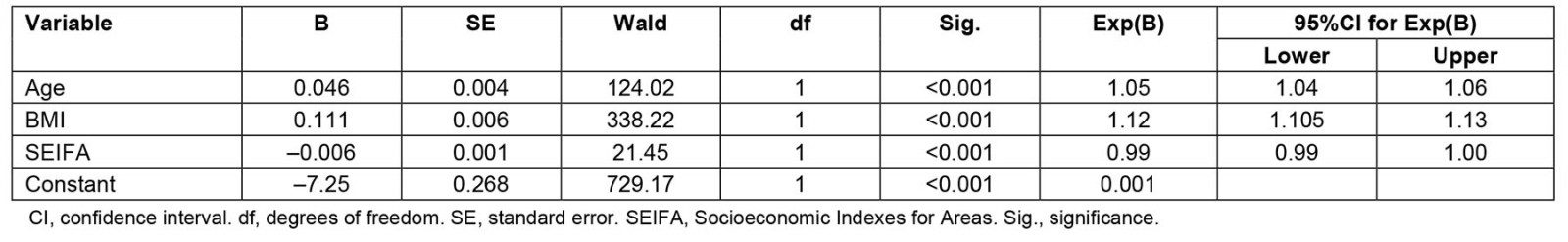
Wave 3 OSA
In wave 3, there was still a significant difference in age, BMI and SIEFA between the different locations (Table 4); however, the rate of diagnosis of OSA during the lifetime at wave 3 was no longer significantly associated with location (χ2 (1)=6.54, p=0.057) or being diagnosed with OSA in the previous 12 months (χ2(1)=5.02, p=0.062). Regression analyses showed that age (p<0.001) and BMI (p<0.001) were the strongest predictors of OSA (Table 5).
Table 4: Metropolitan, regional and rural comparisons for sample (n=7262) from wave 3 of Ten to Men study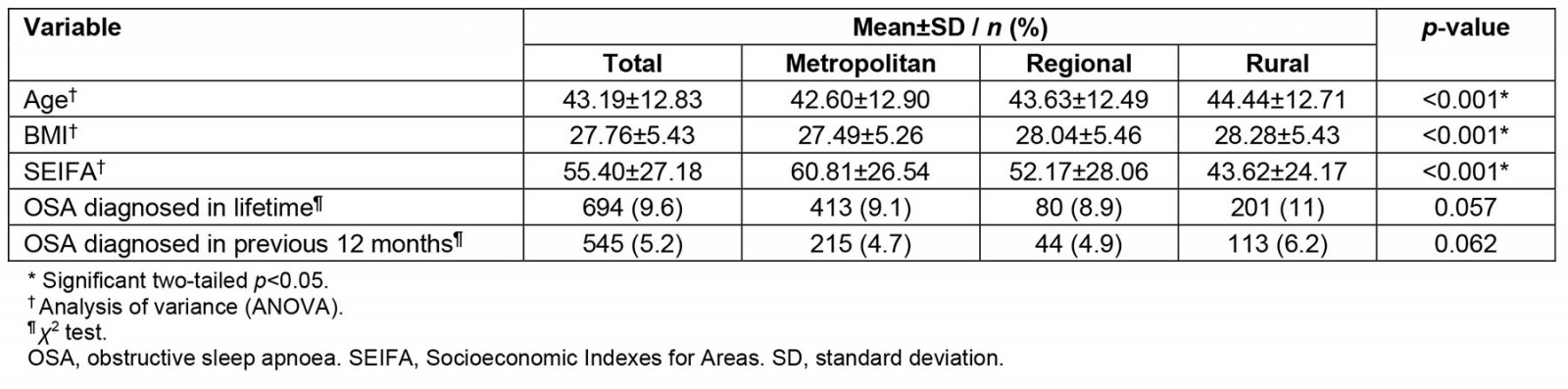
Table 5: Regression analysis exploring factors for OSA diagnosis in a lifetime (yes/no) for sample (n=7262) from wave 3 of Ten to Men study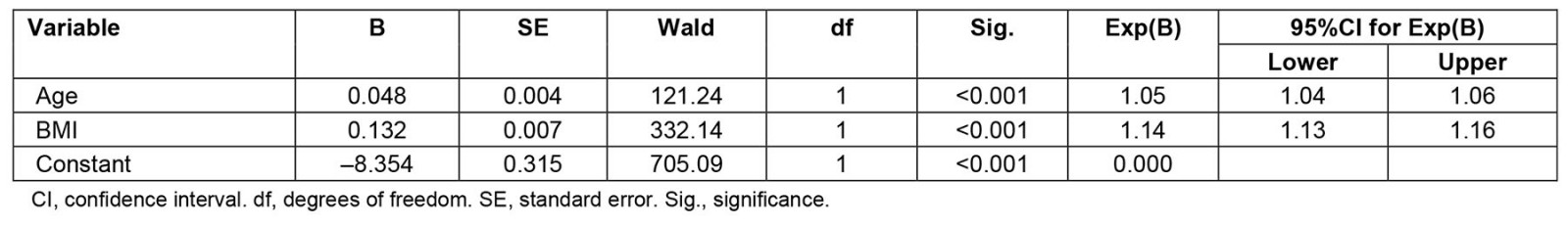
Discussion
The findings partially support the initial assumptions of this study. It was predicted that OSA diagnosis would be lower in rural areas due to a lack of access to services, but this was not the case. The results showed a significantly higher proportion of men living in rural areas being diagnosed with OSA and receiving treatment than metropolitan men, but not in regional areas. In addition, the differences between rural and metropolitan were also consistent across symptoms and risks, including snoring, difficulty breathing and blood pressure, but not fatigue. As a result, a significantly higher proportion of men in rural areas were considered at risk for OSA compared to metropolitan men. Exploration of the factors contributing to an increased likelihood of being diagnosed with OSA demonstrated that, rather than rurality per se increasing the risk of OSA, the differences in the demographic characteristics of populations living in rural areas increased the risk.
In this study the prevalence of men diagnosed with OSA was low (~9%) compared to what would be expected for this demographic (15–40%)8,28-30. While the percentage of individuals with diagnosed OSA was relatively low, the percentage of those with undiagnosed OSA was potentially much higher: approximately 40% of men living in rural locations had signs of potentially undiagnosed OSA, compared to about 32% of those living in metropolitan areas and about 35% for those in regional centres. The higher rate of undiagnosed OSA may explain the low rate of men in rural areas having access to and receiving treatment for OSA, highlighting the need for more targeted services distributed to where they are needed more.
While the differences in the rates of OSA risk and treatment received between rural and metropolitan men highlight a regional disparity, closer inspection of demographic data suggests that this disparity may be attributable to demographic differences rather than rurality. Past research indicates that older age, increased BMI and lower socioeconomic status contribute to OSA7,8,28. However, this is the first research that has explored the intersection between rurality, OSA and OSA risk factors, compared with metropolitan areas. In this sample, men in rural areas were older, had higher BMI and lower socioeconomic status than metropolitan men. After controlling for age, BMI and SEIFA, location was no longer a contributing influence on OSA diagnosis. This finding indicates that the primary driver of higher rates of OSA in rural areas is not rurality itself but rather the demographic make-up of those living in rural areas, making them more likely to experience OSA. Nonetheless, this study found that 1 out of every 2.5 rural men experience symptoms and report risk factors indicating potentially undiagnosed OSA.
However, the difference in geographic location and OSA was narrowly not present in the wave 3 data (p=0.057), even though 11% were diagnosed with OSA in rural compared to 9.1% metropolitan and 8.9% regionally. This wave was collected after the COVID-19 pandemic. It is vital to put these results into context because, during the pandemic, the Australian Government locked down the country, restricting access to only essential services for significant periods during 18 months31. The simple question ‘Have you been treated for OSA?’ may not capture men who have been diagnosed with OSA but have not received treatment yet. Therefore, these figures may underrepresent the possible prevalence of OSA in some areas, particularly given the limited access to services in regional and rural areas. This could have hindered participants from accessing and receiving treatment. In addition, the time between wave 2 and wave 3 was 4–5 years, meaning this cohort of men was older in wave 3.
There are various clinical, health and economic consequences to undiagnosed or untreated OSA32,33. Research shows that individuals with OSA have a greater likelihood of additional underlying comorbidities. Approximately 70% of those with type 2 diabetes have undiagnosed OSA34,35, 40–60% of those with cardiovascular disease have OSA36,37 and 11–18% of those experiencing major depression or suicidal ideation also have OSA38,39. Preoperative screening for OSA shows that 20–40% of patients who were undergoing cardiac surgery were at high risk for OSA40,41. In addition, postoperative complications were up to seven times higher in patients screened as high risk for OSA pre-surgery42. OSA increases the disease severity of depression and diabetes if left untreated16,43,44. Moreover, successful treatment of OSA reduces a range of comorbidities, including night-time glucose levels and insulin sensitivity45,46, coronary heart disease47, hypertension48, depression and mortality rates17,47,48. Considering the critical links between OSA, chronic disease and poor outcomes, improving the availability and nature of the delivery of sleep services to meet the needs of a rural population would help with the associated burden of disease related to comorbidities. Addressing these issues is especially necessary for rural areas, where there are higher rates of chronic diseases such as diabetes, heart disease, mental health and mortality1.
In the present study, regional centres were not significantly different in the prevalence of OSA between either rural or metropolitan areas. Given that healthcare services should reflect the needs of the population they serve, the current research highlights the need to reassess the distribution of sleep-related services. The disadvantage experienced by rural communities is more clearly evident when regional and rural are classified separately in contrast with treating them as the same. Previous research has primarily treated regional and rural areas as one. The presence of health services, proximity of those services and arguably higher socioeconomic status experienced in regional centres are likely counterbalancing the inequity experienced by rural communities. Due to population growth, some regional centres have comparable health services to metropolitan areas. Regional centres could be part of the solution in bolstering rural healthcare support services. A healthcare system whereby larger regional centres are provided with additional services to meet the needs of their own population and deliver innovative outreach services to rural people would help strengthen both regional and rural service provision. The burden of OSA and lack of disease identification and effective treatment in the rural setting are not unique challenges to Australia. There is an ongoing need for innovation in how communities are serviced, as there are shortages of sleep physicians. A potential lack of access to services might result in delayed diagnosis and treatment of OSA, exacerbating underlying comorbidities.
Consequently, by reducing OSA, we could see a reduction in related diseases. The consequences of untreated OSA include an increased risk of high blood pressure, heart attack, stroke and diabetes4, and an increased risk of motor vehicle accidents49,50. Therefore, there should be greater access to sleep services in rural areas, not less.
Limitations
The research team acknowledges that there are limitations associated with secondary data analysis, including not used for its original purpose, being cross-sectional and collected between 2015 and 2016; however, this is a large national dataset, comprising similar proportions of metropolitan, regional and rural participants. This has enabled the comparison of OSA rate and geographical location nationally, which has not been undertaken before. Data for wave 3 were collected during the COVID-19 pandemic, so accessing and getting treatment during this time may have been impacted. Furthermore, we acknowledge that data on neck circumference were not collected in the dataset to measure OSA risk accurately; however, omitting this question and classifying it as a 0 meant that the results underestimated the potential OSA risk and undiagnosed OSA in this population. In addition, there was no data on a formal diagnosis of OSA or the nature of the treatment prescribed, and it is not possible to determine where they were diagnosed. Furthermore, there was a significant drop-out rate between wave 2 and wave 3, and OSA risk was not collected in wave 3; therefore, comparing risk over time was not possible.
Conclusion
This study provides essential knowledge and understanding of geographical location, OSA risk and diagnosis that has not previously been explored. Findings highlight that men in rural areas have a higher likelihood of undiagnosed OSA and are more likely to have been diagnosed with OSA than metropolitan men. While these differences might well be explained by demographic differences in age, weight and income, these findings highlight the need to ensure adequate services in rural areas, given the higher proportion of men in rural areas diagnosed or at risk of OSA. To address this need, a rethink of the distribution of healthcare services is required.
Acknowledgements
The data used in this analysis were gathered as part of the Australian Longitudinal Study on Male Health by the University of Melbourne. Thank you to the men and boys who participated in the study. The Australian Institute of Family Studies manages Ten to Men. Ten to Men research data are the intellectual property of the Commonwealth.
Funding
No funding was received for this study.
Conflicts of interest
The authors declare no conflicts of interest.
References
You might also be interested in:
2021 - Provision of specialized care in remote rural municipalities of the Brazilian semi-arid region
2020 - Community acquired pneumonia: a cost-of-illness analysis in Greece
2017 - Use of telehealth for health care of Indigenous peoples with chronic conditions: a systematic review


