Introduction
Iron deficiency anaemia (IDA) is a common health problem in persons of all physiological groups. However, pregnant women face a higher risk of IDA because additional iron is needed to meet the iron needs of the growing fetus and supply the increased iron requirement for the expanding maternal red blood cell mass1,2. Suboptimal levels of iron during pregnancy are associated with a number of adverse outcomes, including low birth weight, preterm birth3,4 and, in more severe cases, maternal fatality5. Thus, the iron status of pregnant women is widely considered an important determinant of pregnancy outcome around the world.
Reducing the burden of IDA during pregnancy has, therefore, become an important aspect of global efforts to reduce the burden of maternal–infant mortality, especially in rural populations in developing countries where reported figures of maternal–infant mortality remain relatively higher5,6. The strategies widely advocated to address this burden include improved dietary supply of iron, regular intake of iron and folic acid supplements and the periodic evaluation of the iron status of pregnant women7-9. In Nigeria and other countries of the tropics, additional preventive strategies are advocated and implemented for reducing the burdens of endemic parasitic diseases, such as malaria and helminthiasis, which are associated with increased risk of IDA in pregnant women10,11.
In addition to its implications on pregnancy outcome, there are strong concerns that suboptimal iron status in pregnant women also affects the ability of neonates to accumulate sufficient iron stores before birth12,13. This is because the iron endowment of newborns is determined to a great extent by the iron status of their mothers, especially in the second and third trimesters when most of the accretion of foetal iron occurs14-16. These foetal iron deposits are important because newborns rely on them in the first months of life to compensate for shortfalls in their dietary iron intake and to protect against anaemia and its associated far-reaching consequences, which includes the impairment of brain development16. How soon these iron deposits are depleted is determined by the concentration at birth, as well as the dietary iron intake in the early months of life. By inference, children born with inadequate iron deposits would face an increased risk of anaemia in the early months of life, because the deposits may not last long enough to continue to compensate for shortfalls in their dietary iron intake. Another plausible inference is that the risk of anaemia will be higher in exclusively breastfed infants because breast milk is a poor source of iron12.
Although orally administered iron and folic acid supplements are widely recommended for pregnant women attending antenatal clinics, a number of problems, including poor absorption and non-compliance, which could be common in rural dwellers, are believed to affect the effectiveness of these supplements in preventing IDA in pregnant women. Therefore, there is need for studies of this type to determine if the supplements are effective in reducing the burden of IDA. The aim of this article is therefore to determine the iron status of pregnant women attending an antenatal clinic and to ascertain if they face the risk of giving birth to babies with suboptimal iron deposits. This study is important because although prevailing socioeconomic conditions, characterised by poor nutrition and a predisposition to a number of endemic parasitic diseases10, combine to increase the risk of IDA in pregnant women in rural populations, the prevalence and severity of IDA in pregnant women in rural populations of Nigeria and other parts of the tropics deserve further investigation. In this study, we used a combination of haemoglobin (Hb) concentrations and SF levels to determine the iron status of pregnant women attending an antenatal clinic at a public hospital in Nsukka, a semi-urban town in south-east Nigeria. The choice of SF and Hb levels as markers of iron status is informed by their reported suitability for clinical and population-wide studies such as this5,17. Besides, combining both parameters gives a better picture of the iron status of pregnant women than Hb concentration alone, which is commonly used to assess the iron status of pregnant women attending antenatal clinics in rural and semirural populations Nigeria.
Methods
This was a prospective cohort study conducted between April and August 2021 at Bishop Shanahan Hospital, Nsukka, Enugu state, Nigeria. Nsukka is a semi-urban town and the hospital is a public hospital that is run by the Roman Catholic Mission and is attended by rural populations around Nsukka. The study participants were recruited by convenience sampling, and a total of 174 consecutive and consenting pregnant rural dwellers in the second and third trimesters and aged 21–40 years, who met the inclusion criteria, were recruited from the antenatal clinic of the hospital for the study. The gestational stages were obtained from the clinics and were determined through ultrasound. Participants who admitted to being smokers or diagnosed with known conditions, such as sickle cell anaemia, HIV and untreated malaria, which could affect the results of study, were excluded from the study.
Determination of haemoglobin concentration
Hb concentration was determined by the cyanmethemoglobin method18. Anaemia was defined as Hb concentration less than 11.0 g/dL.
Determination of serum ferritin concentration
Serum ferritin (SF) concentration was determined by the enzyme immunoassay method19. The AccuBind Ferritin Assay Kit was used for the determination. An SF concentration of 15 µg/L was used as the cut-off point for iron deficiency. Participants with SF concentrations less than 15 µg/L were diagnosed as having iron deficiency. Such participants were identified and offered treatment in line with the practice of the antenatal clinic.
Diagnosis of iron deficiency, iron deficiency anaemia and non-iron deficiency anaemia
Iron deficiency was diagnosed as serum SF<15 µg/L, IDA was diagnosed as Hb<11 mg/dL and SF<15 µg/L, while non-iron deficiency anaemia was diagnosed as Hb<11 mg/dL and SF³15 µg/L.
Demographics
Demographic data of the participants, including age, stage of pregnancy, consumption of iron supplements and obstetrical history (such as parity and number of previous pregnancies), were obtained through a questionnaire. The questionnaire was written in English and was administered by the researchers with the help of staff of the clinic.
Data analysis
Data were analysed using Statistical Package for Social Sciences v22.0, for Windows (IBM Corp; https://www.ibm.com/products/spss-statistics). Comparison of means was prepared by one-way ANOVA test and Pearson correlation. The test was performed to assess the correlation between the Hb concentration and SF levels with other variables.
Ethics approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki. The procedures involving human participants/patients were approved by the ethics authorities in the Faculty of Biological Sciences, University of Nigeria, Nsukka, and Bishop Shanahan Hospital, Nsukka. Written and signed informed consent was obtained from all participants.
Results
Demographics
The demographic data of the 174 participants are shown in Table 1. The participants were aged 21–40 years. A total of 58 (32.7%) of the participants were in the second trimester, while 116 (67.2%) were in the third trimester. The participants were further categorised in terms of interpregnancy intervals and on the consumption of iron supplements. The most common interpregnancy intervals were 21–40 months (49.2%) and 0–20 months (46.5%). A high proportion of the participants (85.6%) admitted to the regular consumption of iron supplements, while the rest (14.3%) did not.
Table 1: Demographics of study participants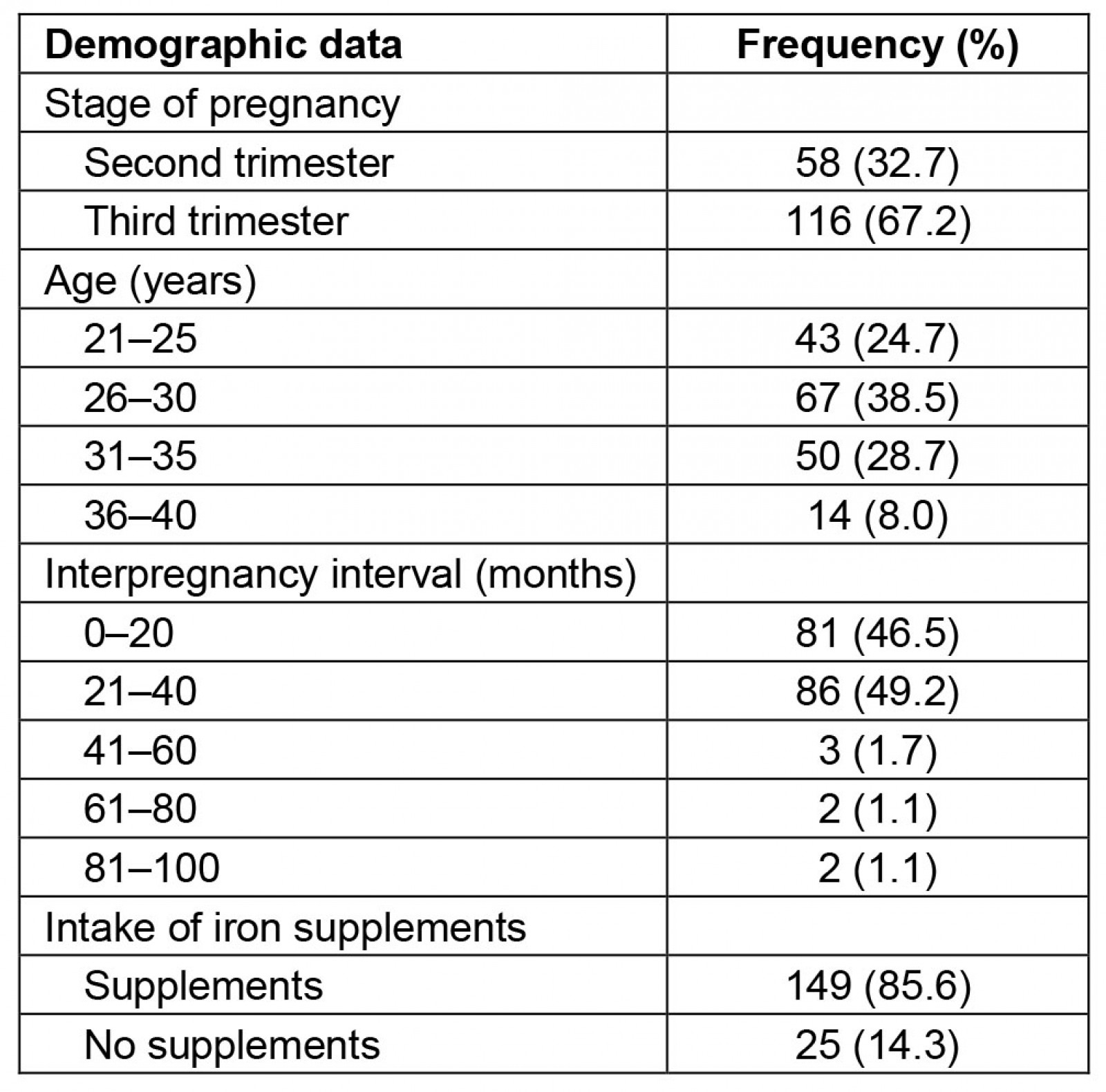
Prevalence of anaemia, iron deficiency, iron deficiency anaemia and non-iron deficiency anaemia
Data on the iron status of the participants are shown in Table 2. The prevalence of anaemia was 48.0%, while the prevalence of iron deficiency was 40.8%. In addition, the prevalence of iron deficiency anaemia was 23.6%, while the prevalence of non-iron deficiency anaemia was 24.7%.
Only 33.9% (59/174) of the participants met the criteria of Hb concentration and SF levels set for normal iron status. A higher percentage (38.8%) of the participants in the third trimester than those in the second trimester (24%) met these criteria.
Table 2: Iron status of study participants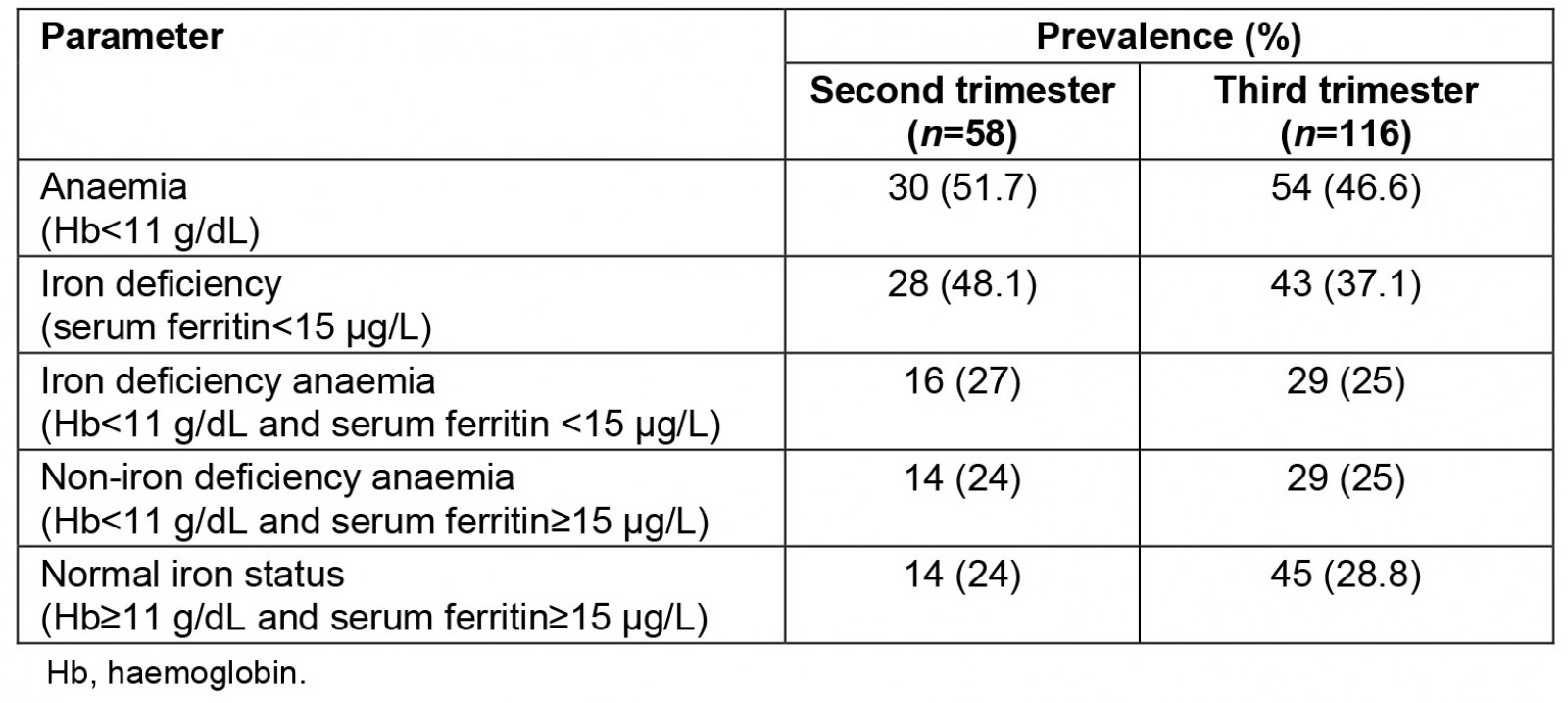
Effect of maternal age, gestational stage, interpregnancy interval and consumption of iron supplements on serum ferritin concentrations
The serum concentration of ferritin varied with maternal age (Table 3). The highest mean SF concentration (41.02±4.01 µg/L) was observed in the 31–35 year age group, while the lowest mean concentration (20.04±3.76 µg/L) was observed in the 21–25 year age group, which was the youngest age group. The mean SF concentration also varied with the gestational stages of the participants. Participants in the second trimester had a mean SF concentration of 43.87±3.76 µg/L, while those in the third trimester had a significantly (p>0.05) lower mean SF concentration of 30.31±3.89 µg/L.
Similarly, the mean SF concentration of the participants varied with the interpregnancy intervals. The highest mean concentration of SF (63.87±4.93 µg/L) was recorded in the group with an interpregnancy interval of 41–60 months, while the lowest mean SF concentration (25.82±2.92 µg/L) was recorded in the group with an interpregnancy interval of 0–20 months. The consumption of iron supplements also affected the mean SF concentration of the participants. The mean SF concentration recorded in the group that regularly consumed iron supplements (37.10±3.02 µg/L) was significantly (p≤0.05) higher than the mean concentration of the group that did not consume the supplements (20.76±2.11 µg/L).
Table 3: Influence of maternal age, gestation stage, interpregnancy interval and intake of iron supplementation on serum ferritin concentrations of study participants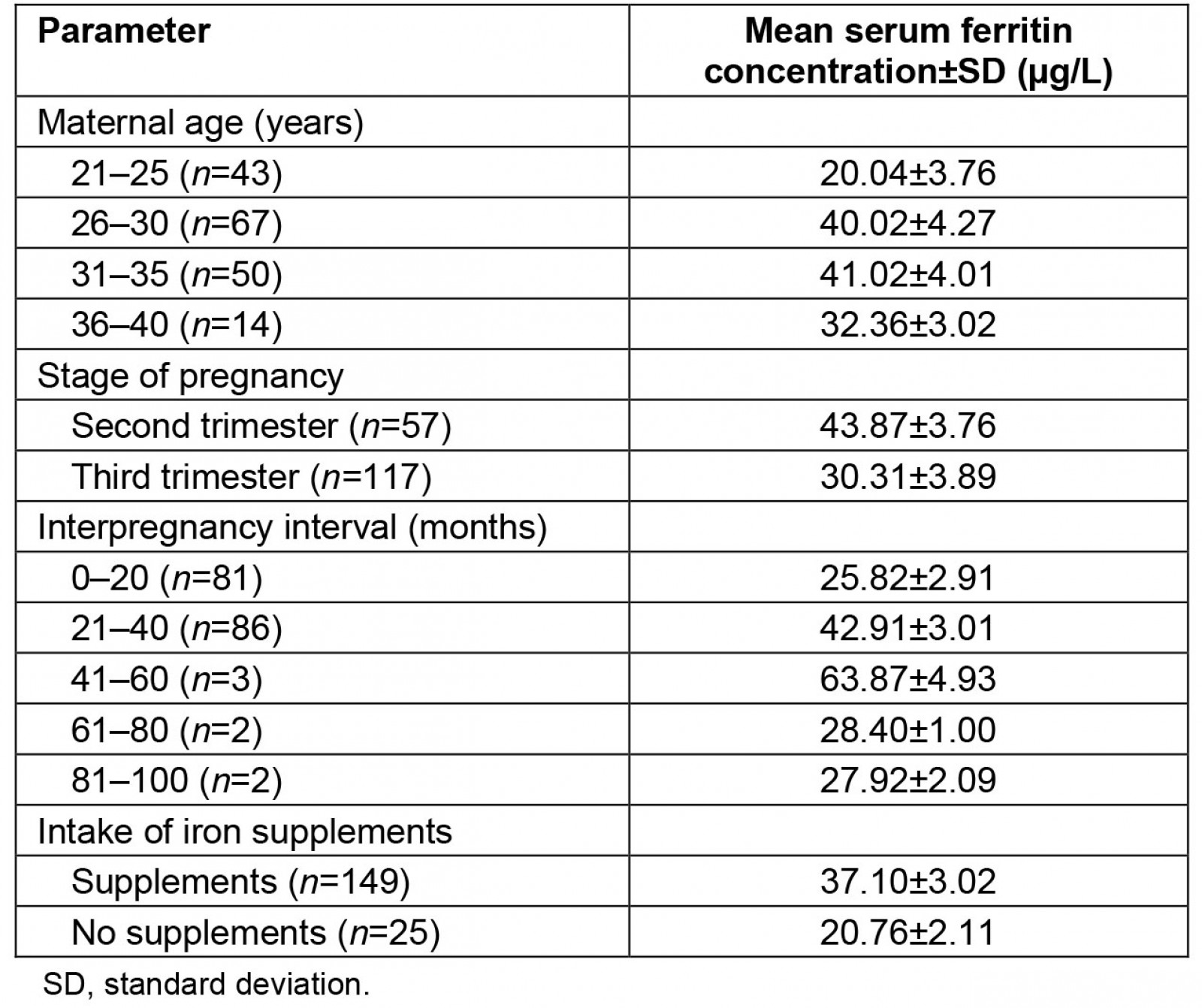
Discussion
This study set out primarily to investigate the iron status of a population of rural Nigerian women in the second and third trimesters of pregnancy, with a view to establishing the percentage of the participants with poor iron status and, therefore, who are at risk of giving birth to babies with suboptimal iron deposits. The study is necessitated by the concern that maternal iron status determines the concentration of foetal iron deposits, which newborns depend on to compensate for shortfalls in dietary iron intake in the first few months of life14. Exclusively breast-fed babies rely on these deposits to meet their daily iron needs since the iron supply from breast milk falls far short of the recommended daily iron requirement at this stage of life13-15.
The 40.8% prevalence of iron deficiency observed in this study is quite high, but is comparable to the prevalence figures reported previously in studies on iron deficiency in Nigeria20,21. Such high prevalence figures are common in rural and semirural populations in developing countries of tropical Africa and are partly attributable to poor nutrition linked to poor socioeconomic status and a higher predisposition to malaria and other infectious diseases, such as helminthiasis22.
The prevalence of 23.6% reported for IDA in this study is almost double the prevalence of 12.3% reported by Ajepe et al21 in a study of pregnant women in an urban Nigerian population. The lower prevalence of IDA reported by Ajepe et al21, could be partly because their study was conducted in the more metropolitan city of Lagos and at a teaching hospital, where people of higher socioeconomic status are more likely to attend. As shown in Table 2, the prevalence of anaemia, ID and IDA were all higher in the second trimester than in the third trimester. This is probably because of the higher rate of consumption of iron supplements in the third trimester than in the second trimester.
The SF concentration of 15 µg/L used as the benchmark for the diagnosis of ID in this study was recently identified as the critical threshold for identifying pregnant women who would be unable to accrete their foetuses with sufficient iron deposits23. Curiously, the SF level of up to 40.8% of the participants fell below this threshold. This implies that babies born to four out of 10 of the participants would be at risk of being born with a compromised iron status. Based on the lower benchmark of 13.6 µg/L recommended by Sweet et al24, the percentage of the participants that would be at risk of giving birth to babies with a compromised iron status would still be as high as 38% (66/174). This implies that a relatively high percentage of these participants would give birth to children with suboptimal iron concentrations. These projections are supported by the findings of a study25 that reported low SF levels in the cord blood of more than half a population of newborns in Nigeria.
The current advocacy for exclusive breastfeeding for up to 6 months is based on the projection that the iron deposits of full-term babies would last this long and will continue to compensate for the deficits in iron intake from breast milk12,13,26. From the foregoing, this may not be the case in a high percentage of babies born in rural parts of Nigeria and other countries of tropical Africa. It is worthy of note that such low levels of SF were observed in the participants in spite of the regular consumption of iron supplements, especially in the third trimester. Our concern about the ability of such children to meet their iron needs when exclusively breast fed for 6 months is strengthened by new evidence that suggests that the concentration and bioavailability estimates previously reported for iron in breast milk may have been overstated26,27. In light of this new information, it can be argued that foetal iron deposits are far more important in supplying the iron needs of babies than was previously thought. This, in turn, implies that foetal iron deposits play a more critical role in preventing anaemia in the early months of life than was previously believed.
About one in four of the participants (24.7%) was diagnosed with non-iron deficiency anaemia, or anaemia of inflammation. The relatively high prevalence of this form of anaemia among the participants is attributable to the endemicity of several inflammatory tropical infectious diseases in Nigeria. The most common of these diseases is malaria, whose prevalence in pregnant women in south-eastern Nigeria could be very high28. In a study on the prevalence of malaria in pregnant women in south-eastern Nigeria, malaria parasitaemia was reported in 99% of the study population28.The plasmodium parasites that are responsible for malaria usually cause the rapid destruction of red blood cells, resulting in a significant reduction in Hb concentrations in the patients. It is possible at such times, therefore, for the rate of destruction of red blood cells to exceed the rate at which they can be replaced. The physiological implication of non-iron deficiency anaemia is not yet known, and it is not thought to adversely affect the iron endowment of newborns29. However, the physiological attempt to restore normal Hb levels in the affected participants would require additional iron and this may have an impact on their iron status.
From the results (Table 2), only 33.9% of the participants met the criteria of normal iron status based on their blood Hb concentration and SF levels. A significantly higher percentage of the participants in the third trimester (38.8%) met this criterion in comparison to only 24% of the participants in the second trimester. The difference between the two trimesters could be attributed in part to the fact that a much higher proportion of the participants in the third trimester (96.5%) admitted to the regular consumption of iron supplements than those in the second trimester (65%).
A number of factors were found to affect the mean SF levels of the participants. Participants in the lowest age group (21–25 years) had the lowest mean SF of all the groups. This is thought to be because this group is more likely to include a higher number of first-time mothers, as opposed to participants in the older age groups, such as those aged 31–35 years, who had a significantly higher mean SF concentration of 41.02±4.01 µg/L. Being first-time mothers implies they may not be aware of the need for additional iron requirements in pregnancy. The gestational stage also affected the mean SF concentrations of the participants. Participants in the second trimester had a significantly (p≤0.05) higher mean SF concentration of 43.87±3.76 µg/L than those in the third trimester, who had a mean concentration of 30.31±3.89 µg/L. Such lower concentrations of SF in the third trimester are thought to be due to the increased accretion of foetal iron, which occurs at this stage of pregnancy. A physiological mechanism that gives priority to the accretion of foetal iron at the expense of maternal iron is thought to operate in humans30. This observation is remarkable considering that a much higher percentage of the participants in the third semester admitted to the consumption of iron supplements than those in the second trimester.
The SF levels were also found to be affected by interpregnancy intervals as well as the consumption of iron supplements. Participants who allowed a longer interpregnancy interval of up to 41–60 months had a significantly (p≤0.05) higher mean SF concentration (63.87±4.93 µg/L) than those who allowed a shorter interval of 0–20 months (25.82±2.92 µg/L). This is because longer interpregnancy intervals allow more time for women to rebuild their iron stores and therefore be more able to meet the iron demands of the next pregnancy31.
The high percentage of the participants (40.8%) with suboptimal SF levels in spite of the regular intake of oral iron supplements casts doubts on the compliance and effectiveness of the supplements in achieving the desired improvement in the iron status of the participants. Adanikin et al32 and Zhao et al33, made similar observations on the ineffectiveness of iron supplements at improving the iron status of pregnant women and their babies. This has been blamed on poor compliance, poor absorption of iron due to morbidities and the possibility of interference by other food components34. A variety of gastrointestinal perturbations have also been associated with oral iron supplements35. The problem of counterfeit and substandard drugs, which is more widespread in rural communities, may also affect the effectiveness of iron supplementation in pregnant women in rural populations. The reported ineffectiveness of oral iron supplementation in preventing iron deficiency and associated anaemia in pregnancy has led to recent recommendations that the intravenous administration of iron be considered for all pregnant women in the third trimester35-38. Such a strategy is needed more urgently in developing countries of the tropics, where a combination of socioeconomic factors and the endemicity of some tropical infectious diseases predispose pregnant women in rural areas to a higher risk of iron deficiency anaemia2,20.
The fact that this study was conducted at a single health facility constitutes a limitation, as does the population size of 174. In addition, participants who did not take supplements were underrepresented.
Conclusion
A very high proportion of the participants drawn from the rural communities were diagnosed with IDA. In addition, the SF concentration in as many as one-quarter of the participants fell below the threshold of 15.0 µg/L recommended for defining those at risk of giving birth to babies with low iron stores. Thus, additional efforts are needed to reduce the burden of IDA in rural Nigerian populations. These efforts need to recognise and address some of the prevailing socioeconomic factors, which include low levels of literacy, poverty, and poor standards of nutrition and hygiene, which are common among rural dwellers in Nigeria and many other African countries. The current clinical practice of relying mostly on Hb levels to monitor anaemia in pregnant women in Nigeria may not reveal the true burden of IDA, and fails to detect the existence of non-iron deficiency anaemia during pregnancy. Thus, in addition to a more effective method of supplementation, we recommend the routine use of both Hb and SF levels to monitor the iron status of pregnant women during antenatal care. The close monitoring of the iron status of exclusively breast-fed children is also advocated in rural populations to prevent IDA and its consequences at this delicate stage of life.
Funding
No funding was received for this research.
Conflict of interest
The authors declare no conflicts of interest.




