Introduction
Respiratory disease remains the predominant reason for paediatric retrievals from remote areas in northern Australia, with conditions that can be defined under the umbrella term ‘acute hypoxaemic respiratory failure’ (AHRF)1,2. Aboriginal and Torres Strait Islander children are four times more likely to have respiratory illness, 34.5% more likely to be moderately unwell and 6% more likely to have a severe form of the respiratory illness compared to non-Indigenous Australian populations3. AHRF has been described as a paediatric respiratory illness that requires oxygen1. Nasal high flow (NHF) therapy provides oxygen or air using a higher flow than standard oxygen therapy1,4,5. NHF therapy helps to keep alveolar sacs – which may have otherwise closed – open in between breaths. This reduces the amount of dead space within the lungs and a greater ability for the perfusion of oxygen6,7. This higher flow can reduce the amount of oxygen needed, and in some cases it may conserve oxygen resources where high flow room air is sufficient in improving the patient’s condition8. NHF therapy humidifies the flow of air–oxygen blend and reduces the mucous membrane damage that is normally expected with any form of unhumidified oxygen1,8.
NHF therapy is easy to apply; however, the clinician must be skilled in caring for the unwell child7. This is a challenge in remote locations, where nursing and medical clinicians are rural generalists and specialist paediatric services are often hours away, with aeromedical services required if a patient needs specialist care. The use of NHF therapy in tertiary hospitals throughout Australia is ubiquitous, attributed largely to the Paediatric Acute Respiratory Interventions Study (PARIS) group, who in the past decade have conducted numerous studies including the largest randomised controlled trials in children with respiratory illnesses presenting to emergency departments in Australia and New Zealand. These studies have provided clinicians with much-needed answers about how to best use NHF therapy in children9,10. In remote hospitals throughout Australia, the implementation of NHF therapy is inconsistent, without clear guidance of its use in this limited-resource environment. In Torres and Cape Hospital and Health Service, where this study took place, the three main remote hospitals all reported significant discrepancies in its use from ‘never used’ to ‘regularly used’, depending on the medical officer’s experience with the therapy. It is likely this is a similar experience across much of remote Australia.
Differences in healthcare access exist within remote communities compared to tertiary settings11,12. Remote populations13 and health staff consider larger contextual complexities when making decisions around health care, such as impact of retrievals on the family and the health service, both psychosocially and financially14,15. Clinical guidelines promote evidence-based practice and assist clinicians to make decisions regarding patient care for specific clinical circumstances16. However, NHF therapy clinical guidelines in remote Australian locations17 are not tailored to the geographical remoteness of the location and offer few suggestions on how the guideline should be applied in this context. Health inequities can be partially addressed by developing clinical guidelines where recommendations are specific to disadvantaged subgroups, such as those with geographical difficulties in accessing health care18. Development of guidelines and the use of the guidelines are not the same; guideline developers should consider the feasibility of implementation when making recommendations16. Context-specific clinical guidelines have the potential to reduce resource wastage and improve patient outcomes16,18,19. A clinical guideline for the use of NHF therapy in the three participating remote hospitals was published on 28 October 2021 and is currently in use (Supplementary material 1)20.
The aim of this study was to develop a guideline for the use of NHF therapy in three remote hospitals in Queensland, Australia.
Methods
Study design
A modified Delphi technique was used to reach consensus on the guideline21-23. Delphi techniques are particularly useful to develop guidelines in clinical settings where there is minimal existing literature in unique contexts such as rural and remote hospitals22,24. A Delphi technique typically requires expert opinion, anonymous participation and measurable feedback, and outcomes are iterative22. This technique was modified to include roundtable discussion via videoconference, interspersed with rounds of draft guideline statements where participants replied with full, partial or no agreement until final consensus was reached. This study is part of a larger research project exploring the implementation of NHF therapy in rural and remote hospitals.
Setting
Cape York and the Torres Strait Islands are very remote tropical regions in Far North Queensland. Sixty-five percent of Cape and Torres Strait residents identify as Aboriginal and/or Torres Strait Islander25, which is considerably higher than the rest of Queensland, at 3.6%26. With an area of 220 000 km227,28 and a very dispersed population of 26 000 people26, this region has four very remote hospitals (Modified Monash Model rural classification of MM 6–7)29. One of the four hospitals is accessible year-round by road, two hospitals are inaccessible by road during the ‘wet’ tropical season and the fourth hospital is located on an island. The Clinical Services Capability Framework (CSCF) is a tool to stratify resources, available services, capacity and capabilities of each health site across Queensland25. The higher the CSCF grading, the more capability a site has in providing clinical services25. Each different service receives a grading at each health site25. Three of the four included hospitals are a CSCF 3 in paediatrics and emergency, the fourth hospital is a CSCF 2 and was not included in this study (tertiary settings would generally be graded CSCF ≥4)25. The facilities are staffed with highly mobile rural and remote clinical staff, with a high turnover of agency/locum workforce. All facilities are open continuously, with an emergency department on skeleton nursing staff after hours, an aged care facility and an inpatient ward (22 beds in Weipa, 32 beds in Thursday Island, 29 beds in Cooktown)25. Medical officers work on an on-call basis after hours. The closest paediatric intensive care unit (PICU) is a helicopter and aeroplane flight away in Townsville (451–1083 km away), and there is a specialist paediatric ward in Cairns (328–802 km away). All these locations require aeromedical retrievals for transfers to tertiary hospitals and are susceptible to inaccessibility and delays in retrieval due to tropical weather events30.
Participants
The expert panel comprised 11 participants: three expert rural and remote doctors (one from each study site); three expert nurses (one nurse educator from each study site); a tertiary paediatric intensive care doctor from the closest receiving tertiary facility with a PICU; a paediatrician from the closest receiving tertiary paediatrics unit; a retrieval medicine doctor; a nurse expert in retrievals, PICU, research and NHF therapy; and the Executive Director of Medical Services, who chaired the meetings. The experts were recruited by Queensland Health clinical leaders. Each panellist was provided with a written participant information sheet beforehand, and all participants provided written consent to participate. All participants contributed to every decision made in the guideline until a consensus was made.
Procedure
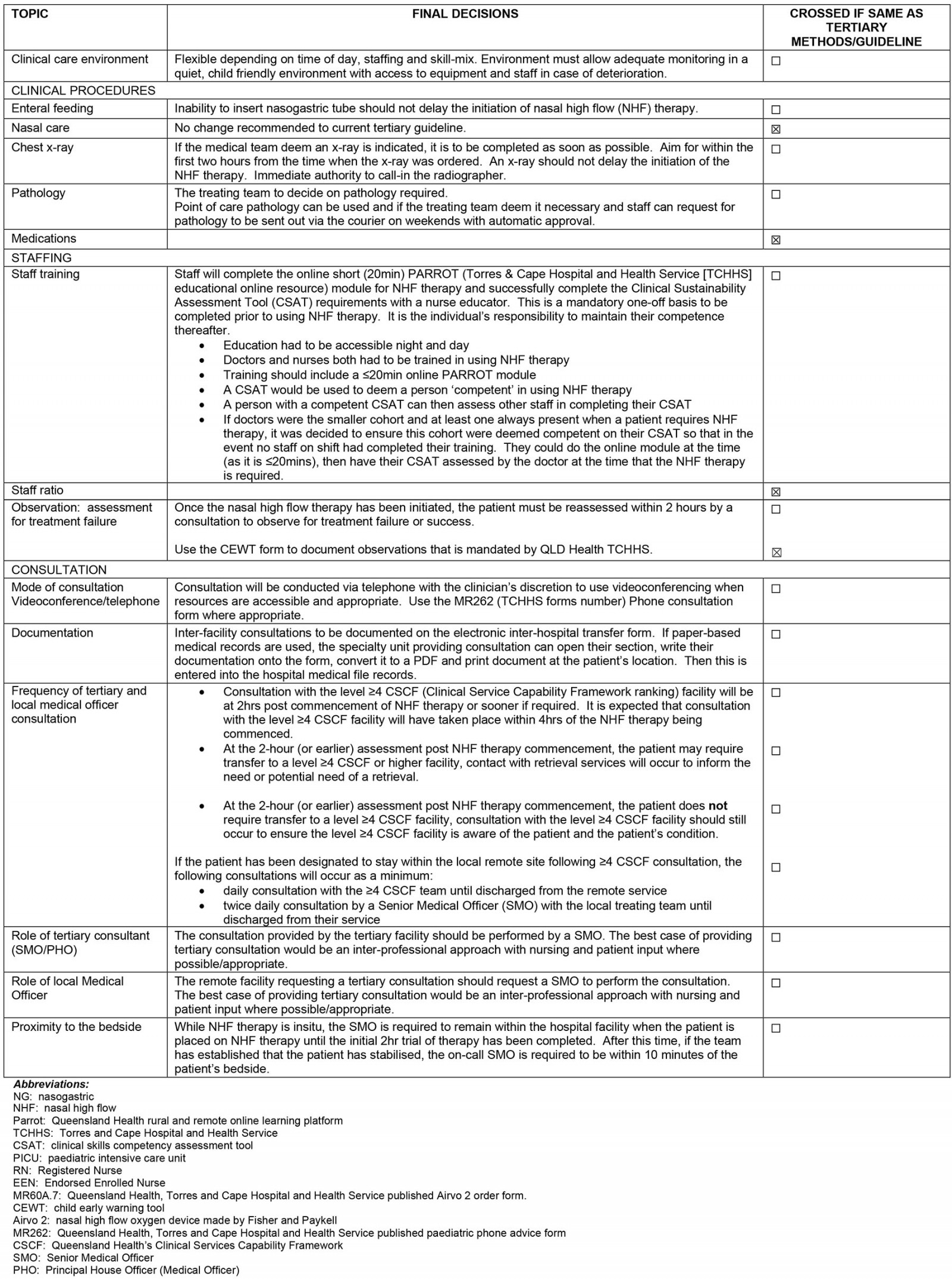
Prior to the first meeting, a summary of searched literature exploring the implementation of NHF therapy was provided to all members of the expert panel (Fig1). The executive summary made recommendations on key discussion topics, including the environment of care, clinical procedures such as enteral feeding, staff ratios and training, and observation and consultation processes. Panel members added discussion points to the meeting, including how to document interfacility consultations and patient flow through the facilities. Participants were provided with existing Queensland Health clinical guidelines for the use of NHF therapy20. The panel did not consider matters that were already detailed in the existing clinical guidelines and were universally applicable between tertiary and remote sites, such as oxygen flow rates. The roundtable discussions were open to all participants with a risk of competing personalities overpowering the discussion. The panel was chaired by the Executive Director of Medical Services, who lived and worked in the remote health service and ensured conduct was fair and adequate for all participants (Fig1). A key points and decisions document was developed from the transcript and other participants emailing their opinions as a method to integrate participants into the discussion.
Due to the location of the participants and difficulty in scheduling, it was decided that all meetings would be held by videoconference. The Delphi technique was modified because contributions were conducted without anonymity to the fellow participants22. Using a modified Delphi technique, the absence of a participant’s opinion/discussion was considered as being agreeable to the decision under discussion. During the expert panel meetings, a subgroup comprising the three participating nurse educators was formed in addition to the existing meetings, to provide detailed recommendations for training requirements. Four meetings were held in total: two iterative Delphi rounds with the whole group, one with the nurse educator subgroup and one with two members who were unable to attend one of the meetings. Every subsequent meeting included re-orientation and updating on key points from previous meetings (Fig1). Discussions focussing on topics that would not change from the tertiary context to the remote contexts were redirected.
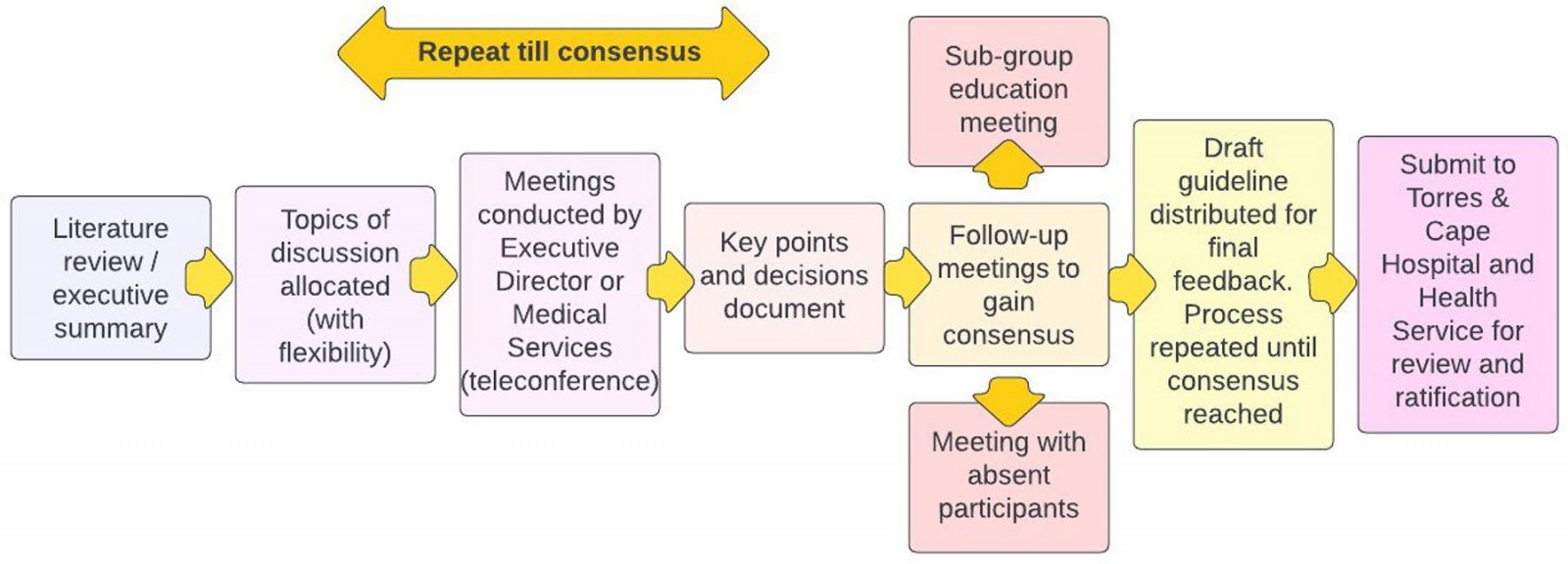 Figure 1: Flowchart for guideline development using the modified Delphi technique.
Figure 1: Flowchart for guideline development using the modified Delphi technique.
Guideline development (data analysis)
The visual flowchart (Fig1) describes the following processes. Meetings were held, with the panellists referring to an executive summary. The meetings were videorecorded, transcribed verbatim and the recommendations synthesised into a decision document. The remote specific guideline was developed iteratively to build consensus. Comments relating to each key topic were summarised, duplicate comments were removed, key comments were retained and a final decision was listed once participants had reached consensus. Final decisions were added to an amended guideline document. The decision document provided a chronological summary of how the decision was derived. The decision document was provided after each meeting to allow participants to report full, partial or no agreement, and to add further comment(s) either via email or at the next meeting. Expert opinion on the implementation of NHF therapy was also sought during this process. The final amended guideline was submitted to the Torres and Cape Hospital and Health Service’s clinical education team for review and ratification through their clinical governance unit.
Ethics approval
This study received ethics approval through the Far North Queensland Human Research Ethics Committee (HREC/2021/QCH/54605).
Results
Final decisions relating to the implementation of NHF therapy in the three remote hospitals are presented in Appendix I. Four out of 18 final decisions were not altered from the tertiary guidelines because practice was evidence-based and appropriate for the remote context. The four unaltered decisions related to using the Child Early Warning Tool for observations, and to nasal suctioning, medications and staff ratios. The results presented below are a summary of the key issues raised by the expert panel that were incorporated into the clinical guideline.
Clinical care environment
The care environment might change depending on clinical need, with one nurse stating:
If a ward patient deteriorates and is thought to require NHF, they must be escalated appropriately. This includes moving to an area where closer monitoring is possible, such as return to ED, or move to a single patient room closer to the nurses station. (Nurse, remote)
Clinical procedures
Remote site participants reported that there would be medical and nursing staff that rarely had to insert a nasogastric tube and may feel inexperienced or uncomfortable with performing this procedure. There were concerns that the requirement of inserting a nasogastric tube would delay other aspects of patient care.
The importance of incorporating education on nasogastric tube insertion as part of the NHF therapy protocol was recognised. All panel members agreed that nasogastric tube insertion hesitance should not delay the use of NHF therapy and, if necessary, staff competent to insert the nasogastric tube should be called into the hospital out of hours.
A key issue was that after-hours organisational processes, which may take longer in the remote hospitals, should not delay treatment to NHF therapy nor affect patient safety. Panel members agreed that 2 hours was a minimum standard, with the recommendation that performing the X-ray should not delay NHF therapy initiation, and call-in approvals would no longer be required. Timely access to pathology results involved approval for pathology to be sent to the nearest processing town on weekends, instead of waiting for Monday. The process for patient review and consultations with paediatric specialists and retrieval services is summarised in Figure 2.
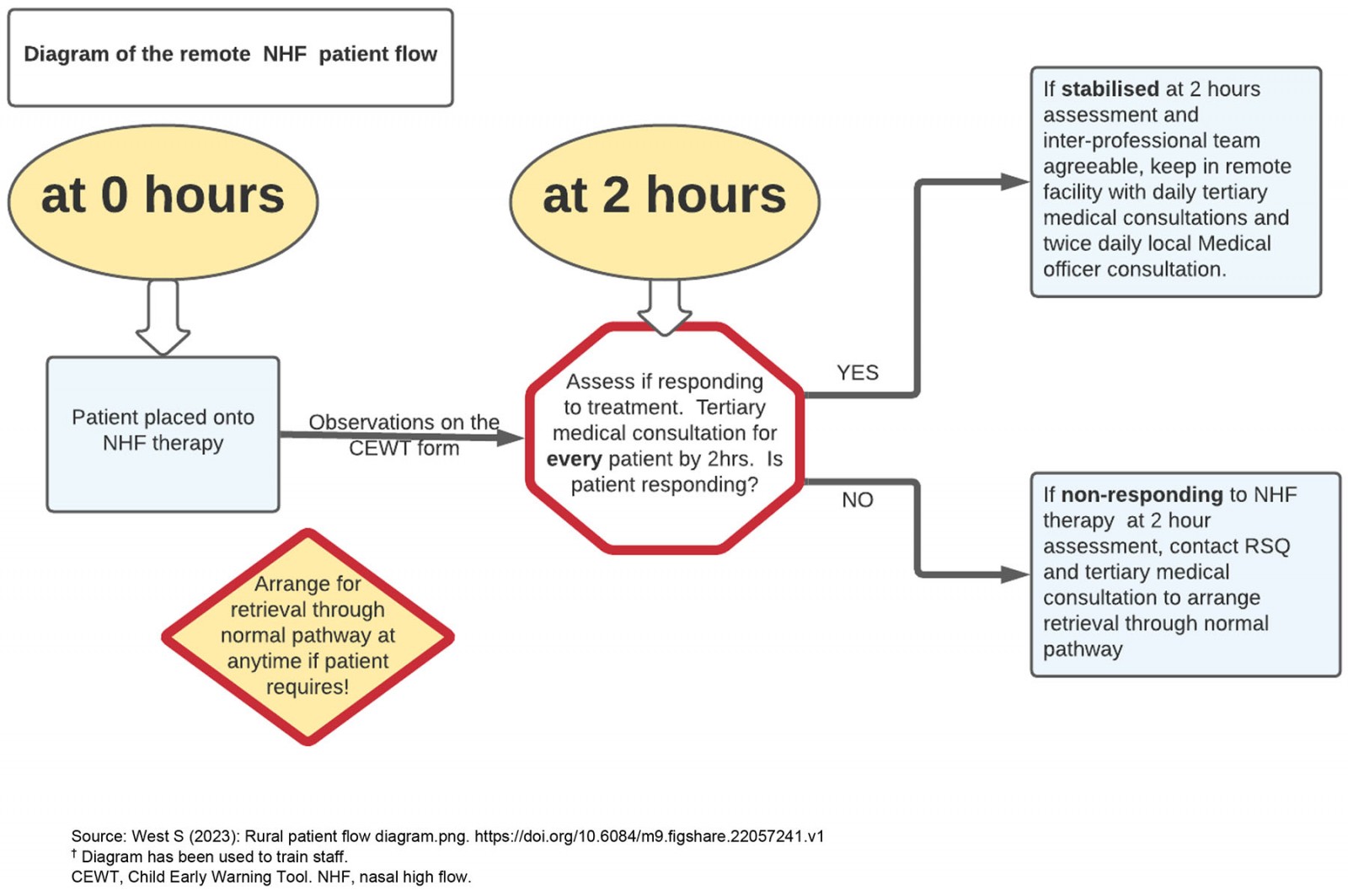 Figure 2: Review and consultation flowchart.†
Figure 2: Review and consultation flowchart.†
Staff training
All participants agreed that a skilled workforce requires both the confidence to use an NHF device and confidence to manage an acutely unwell child.
… it’s not just the set up and the initial parts, it’s the recognition that you know, if the patient isn’t getting better within a certain timeframe … So recognition of the deteriorating patient. (Nurse, retrievals)
Staffing ratio
Staffing ratio and skill mix were extensively discussed, and all participants agreed that the workforce requires the confidence to use both an NHF therapy device and to treat an acutely unwell child. This discussion included the ward/department in which the patient should be cared for when receiving NHF therapy, if staffing ratios needed to be prescribed, and how training could be provided to suit the remote workforce with high staff turnover and a large agency workforce. Where patients will be treated (emergency department or ward) needs to be flexible depending on clinical need and staff skill, with one nurse stating:
… we used to teach it was definitely doing 1 to 4 ratio … you’ve got these two babies …they’ve both got sats [oxygen saturations] of 90%, they’re both working hard, they’re both tachycardic, tachypnoeic. They look exactly the same … But the only difference in your care was one had a nasogastric tube … You both did obs every hour, you were both constantly observing that child … If they’re deteriorating, I would go 1 to 1. (Nurse, tertiary)
Staffing ratios were a contentious issue. Some panel members thought the ratio should be determined by the illness of the patient and not the treatment. This view was challenged by the remote health staff who felt that children on NHF therapy would initially require 1 : 1 nurse-to-patient ratio due to the added resource-consuming nature of these patients in the remote setting and small numbers of staff in the facility that can support more than one unwell patient. Rural and remote clinicians emphasised the additional responsibilities required by the rural and remote context, and the cognitive and task load this adds to the clinicians.
… 1 on 1 keeps us safe because you don’t know how they’re going for those first, four, six, twelve hours. That for me is that critical period prior to stabilization and turnaround or deterioration. (Doctor, remote)
Continued disagreement around this topic existed between participants. The panel consensus was to keep the ratio recommendations broad and based around the individual patient requirement. This is unchanged from tertiary recommendations.
Consultation
One tertiary expert and two remote experts emphasised the need to ensure the child who lives remotely receives the same standard of care as what they would in the tertiary setting. Consultations both from the tertiary and the remote end need to be at the senior medical officer level and multidisciplinary.
They would normally see a Consultant Paediatrician. They’re probably reviewed a couple of times a day by a paediatric trainee and are looked after by paediatric-specific nurses 24/7. Which is not to in any way denigrate the care that might be given elsewhere. But I think that if this is the child with the same problem … we’re trying to think, can we provide the same level and interest of care in a different location, not suggest you get something else. (Doctor, tertiary)
Twice-daily local senior medical officer review and once-daily consultation with a paediatric specialist was considered sufficient. A 2-hour timeframe to reassess whether the treatment has succeeded/failed was agreed to allow enough time for the therapy to work but also to ensure there were no delays in identifying the deteriorating child and/or delaying the decision to retrieve the child.
With a 2hr trigger to Retrieval Services Queensland [RSQ] to update on where you’re at with your high flow kid, you can bring them into the conversation and you usually discuss with PICU again or paediatrics at that point to bounce your ‘this is how we’ve gone in two hours, I think we’re looking ok, or we’re not looking OK’, include RSQ. (Doctor, remote)
Senior medical officer proximity to the bedside was agreed to be 10 minutes away as per on-call guidelines once the initial consultation had been conducted.
10 minutes is quite appropriate for the stable [patient]. So we’ve started the treatment, it’s stabilised, we’re happy we know where we are, it looks good. That the nursing staff are comfortable – that is really vital to that decision – then I think 10 minutes is completely acceptable given allocation resources and how it works. (Doctor, tertiary)
Six doctors commented that technical aspects of videoconferencing were not sufficient to mandate it for every consultation.
… we use the most expeditious means to communicate. I don’t mind the telephone and in fact some of the videoconferencing equipment is so far from the child and in fact you struggle to see the little person at the end of the bed. (Doctor, tertiary)
Two tertiary doctors felt that documentation of the tertiary consultation was a significant gap in the current operations of the patient consultation process. A solution provided was using an already existing Queensland Health Inter-hospital transfer form even in the event the patient is not recommended for transfer out.
I think it’s really important and should be documented … the electronic inter-hospital transfer form, it is on all of the desktops throughout Queensland Health. It has an ICU section apart from the transfer section and that could be stood up as an ICU consult and printed out at the end of the day as a PDF document off the computer. (Doctor, tertiary)
Discussion
Providing the remote population with access to the same standard of care that would be received in the tertiary setting is important, but it is not a matter of simply replicating what is performed in the tertiary environment19. Equity in the remote setting looks different to tertiary environments, and for this reason guidelines should be based on how they are to be used in the remote context, to ensure the guideline is feasible and fit for purpose16. Remote hospitals have to deliver care differently compared to other, more populated contexts31. The National Strategic Framework for Rural and Remote Health suggests that rural and remote context problems be solved at the local level where the greatest understanding of the context/problems are based31. Composition of a guideline by an expert panel that includes local representatives is potentially translatable to other remote hospitals and other clinical topics because it first identifies the contextual factors, then the experts decide on the best method to mitigate the context-specific risks32.
One of the main drivers for this study was to ensure safe, consistent and equitable access to paediatric respiratory care between remote sites and, as much as feasibly possible, between tertiary and remote hospitals. Remote health services experience considerable difficulties in translating best practice, designed in tertiary settings, to their contexts11. It became clear during the roundtable discussion that key issues limiting the feasibility of using NHF therapy in remote hospitals were system-level operational issues that potentially slowed treatment commencement, such as the requirement for clinicians to seek approval prior to arranging logistics for required services such as pathology and medical imaging after hours. These are barriers that are not faced in continuously staffed tertiary hospitals. The inclusion of recommending automatic approval for after-hours requests reduces the cognitive burden on the clinicians but also makes the guideline more feasible in its implementation33.
Communication between remote clinicians, retrieval services and the tertiary specialists, including a shared patient information system, was discussed by the panel. If a tertiary consultation was undertaken, but the patient was not retrieved, participants found that there was no reliable system that allowed for tertiary documentation to be entered into the remote hospital within the patient’s medical file. Current systems between these settings can be disconnected, with incompatible health record processes across the tertiary and remote sites34. Tertiary hospitals in Queensland use electronic medical records whereas the remote sites in this study used either paper-based medical files or a different electronic medical record, therefore contributing to a greater risk of information sharing barriers35. While the experts were able to agree on a local mitigating solution by using an inter-hospital transfer form for consultations, there is no ease of use or prompt to ensure that this happens with every consultation, whether there is a transfer or not. Patient safety is compromised when communication between service providers is ineffective36. Integrated electronic patient records still do not support the remote-to-tertiary interface and would warrant ongoing necessity for investigation and investment34.
The feasibility of the use of clinical guidelines must be considered throughout development. It is paramount that the remote-context barriers and enablers are identified and addressed when incorporating a guideline16. Delivery of any therapy in remote settings requires constant consideration of workforce skills and capacity12. One of the greatest barriers to local remote health services providing treatments such as NHF therapy is the lack of staff capability and capacity to not only administer the therapy but also have the confidence to manage the unwell paediatric patient7,33-35. Ensuring staff are available on every shift who are competent and confident in acute paediatric care is extremely challenging in remote hospitals. The high turnover of nursing and medical staff in rural and remote health services, and the generalist skill set required, contributes to difficulties in workforce capability12,37. To mitigate these challenges, the expert panel discussed two key areas: accessibility of education and training and nurse-to-patient ratios. Education and training were simply resolved through recommendations for education and training opportunities to be continuously available across a range of platforms. The nurse-to-patient ratio was a more contentious issue, with considerable debate. Historically, most of these more acute patients have been retrieved to the tertiary centres, reducing opportunities for remote clinicians to use acute care skills. As well as the infrequency of acute paediatric patients, the generalist nature of the work in rural health, extra requirements for non-clinical tasks and reduced support during night shift all increase the cognitive load on rural and remote staff when caring for acutely unwell paediatric patients12,14. Participants had to reframe how they would approach the recommendation and decide on staff ratios based around the wellness of the patient and the workforce or environmental factors at the time. Expectations of the capacity of rural health staff should reflect the expectations of staff being generalists, compared to tertiary paediatric health staff, who solely treat unwell children14,38. Generalist remote nurses may be reflected in increased nurse-to-patient ratio recommendations if needed. The expert panel decided the team should have autonomy to calculate their nurse-to-patient ratio dependent on the degree of illness and activity requirement in real time at every acute paediatric presentation.
The geographic distance to tertiary or specialist health care is an ongoing challenge experienced by remote communities13,39,40. Access impacts on health outcomes and communities, and health disadvantages increase with greater geographic isolation13,15,41. The capacity to access what is needed, when it is needed can be a determining factor for people to remain living in remote communities39,40. The proximity of the remote patient from a tertiary centre where paediatric specialist and intensivists are available greatly influenced the way decisions were made in this study. Retrieving unwell patients to tertiary hospitals instead of upskilling rural staff is one solution, but this process introduces variables, including unpredictable timeframes for retrieval, that may leave the remote patient and hospital exposed, high healthcare costs and importantly the human factor of psychosocial difficulties for families. The panel members, influenced by community and staff feedback, came to the study with the belief that it was not appropriate to ‘fly out’/retrieve every infant who may require NHF therapy, as previous guidelines had recommended42, requiring staff to simply ‘do the best that they can’ while waiting for retrieval services. Retrievals may be delayed for hours or days for any number of reasons, including cyclones, which are a common occurrence in the ‘top end’ of Australia43. Retrievals also add a considerable extra financial and emotional burden onto families, with infants requiring a parental escort, and other care arrangements required for siblings or other loved ones for an unknown number of days44,45. NHF therapy can provide stabilisation of patients prior to retrieval, improve patient safety or potentially reduce the need for retrieval altogether46.
Retrievals are and will always be one ‘tool’ to support health care in rural and remote communities; however, this study focused on improving access to treatment irrespective of whether retrieval services were available or used39,47. Future research that integrates and facilitates the implementation of best-practice respiratory emergency care in remote communities will provide much-needed safe and consistent access to services and increased resilience of service models.
Limitations
Suggestions for future iterative processes included a method that allowed for anonymous and/or private contributions by the panel in real time during the discussions. More time should be dedicated to allow the participants to meet in the iterative Delphi rounds. Increased total group Delphi meetings may have avoided the need for subsequent Delphi rounds to be conducted by email. This modified Delphi technique involved small group of participants within these regions. It may be possible that different people within the participating organisations may have different opinions regarding NHF use in the remote hospitals. It was outside of the scope of this research project to do a broader staff survey on the implementation of NHF therapy in remote hospitals; however, this will be explored in planned subsequent research projects. This guideline was developed for a specific remote region in Queensland and the findings may be different in other contexts.
Conclusion
This study developed a guideline for the use of NHF therapy in three remote hospitals in Queensland, Australia. An expert panel of health professionals met regularly to discuss the Queensland tertiary guideline and determine the suitability of this guideline to the remote context. Of the 18 concepts discussed in the tertiary guideline, only four remained unchanged; these four concepts were deemed suitable to the rural context by the expert panel. The key areas of recommended change by the expert panel were in relation to which ward or department unwell patients should be cared for if receiving NHF therapy in the remote context, providing education to remote staff to increase competence and confidence in skills and knowledge, improved processes for timely access to pathology and imaging requests at these sites, and improved processes for communication and documentation between tertiary and remote sites. These recommendations, which were ratified and published by Queensland Health, aim to promote consistent access and use of NHF therapy between remote hospitals, with the aim of reducing delays in accessing treatment and providing equitable care, despite a person living in a remote setting.
References
Supplementary material is available on the live site https://www.rrh.org.au/journal/article/8516/#supplementary
You might also be interested in:
2013 - Comment: Cost and returns related to medical education in rural and remote locations