Pathology and laboratory medicine are essential components of effective cancer care. Histopathology is the gold standard for cancer diagnosis; the contents of a histopathology report are geared towards informing patient care. Cancer stage and grade, assessment of surgical margins and assessment of other histological features that portend worse outcomes are all determined by histopathologists. Furthermore, molecular markers of response to targeted therapies are often measured in tissue submitted for histopathology, and molecular pathology has become an essential part of modern pathology practice. Despite this central importance of pathology in cancer care, laboratory medicine is understaffed and underresourced globally. This paucity of capacity is amplified in rural and remote healthcare settings and contributes to inequalities in rural cancer outcomes1.
Over the past two decades digital pathology has emerged as a novel technology in histopathology. Instead of viewing glass slides using a traditional microscope, pathologists now have the option of scanning the same slides at high resolution, making whole slide images (known as whole slide imaging, WSI) and viewing these on a screen. The digital slide can be viewed anywhere with a suitable internet connection, and this has proposed benefits in safety, quality and efficiency2. For example, second or specialist opinions can be sought more easily and the removal of physical slides from a laboratory workflow may reduce administrative burden and errors owing to lost or misidentified slides. Digital pathology initially found uses in teaching, external quality assurance and intradepartmental review for multidisciplinary team meetings. Subsequently it has been validated as a safe alternative for primary diagnosis3,4. The COVID-19 pandemic acted as a catalyst for primary digital reporting as pathology departments looked for ways to maintain diagnostic capacity while ensuring social distancing5. In addition, digital pathology can facilitate a hub-and-spoke or networked model of pathology6 (Fig1).
Digital pathology therefore can remove the need for colocalization of pathologist and pathology slide. This has obvious applications in the rural and remote healthcare setting, where distance from and access to specialist services may be limited. In this issue of Rural and Remote Health, Kawakami et al report the implementation of WSI and remote pathologist review for intraoperative frozen section diagnosis of sentinel lymph nodes in breast cancer7. Following the Fukushima triple disaster of an earthquake, tsunami and consequent nuclear disaster, this region suffered well-documented severe consequences including 18 000 deaths and the displacement of 150 000 people8. Among the deleterious effects on healthcare provision was a reduction in pathology workforce. Using WSI, frozen sections could be assessed by pathologists at a hospital 5 km away, thus maintaining breast cancer surgery in the local hospital.
Frozen section diagnosis is one of the most challenging tasks in diagnostic histopathology. A diagnosis must be made under time pressure, using slides whose preparation is suboptimal owing to rapid fixation and processing. Furthermore, a frozen section diagnosis often has an immediate and profound effect on patient care because it informs the extent of surgery. In lung cancer surgery, the frozen section result can determine if a patient has a small wedge of lung removed or an entire lobe, highlighting the importance of this element of pathology9. Frozen section of sentinel lymph nodes in breast cancer is established practice and can determine if a patient requires axillary lymph node dissection intraoperatively, potentially sparing the patient a second operation10.
In their study, Kawakami et al demonstrated good concordance between glass and digital slide diagnoses7. Crucially the authors compared diagnoses from digital frozen section with those from the corresponding traditional light microscopic interpretation and not simply the final diagnosis. This is a subtle yet important point: when making the final diagnosis the pathologist has the benefit of seeing the entire specimen in optimally fixed and sectioned slides. Therefore, comparing this with one frozen section slide is not a fair assessment of the microscopy method used to interpret the frozen section. Similarly to other work in this space11, Kawakami et al showed that it is the challenges of frozen section diagnosis itself rather than the digitization of slides that leads to most discordant diagnoses.
The data reported by Kawakami et al mirror that of previous studies. One of the largest examples of a remote intraoperative consultation is the Eastern Québec Telepathology Network12. This long-running initiative provides frozen section diagnosis in a sparsely populated region where nearly 30% of hospitals do not have a pathology department. Reporting of frozen section WSI images by pathologists more than 200 km from the operating theatre showed good concordance with glass slide diagnoses across a range of specimen types, including lymph nodes in lung and breast cancer, and surgical resection margins. A recent report of more than 2000 cases over 12 years showed similar concordance after slide scanning and remote diagnosis was implemented in South Tyrol in Italy, linking three peripheral hospitals with a central hub over distances of up to 75 km13. Both studies showed good concordance between digital and traditional glass slide diagnoses. A further study demonstrated similar concordance in the frozen section setting and contains a comprehensive literature review11.
Rural and remote cancer care takes place in a variety of contexts, economies and levels of health resource. In high-income countries patients in rural areas often have worse cancer survival compared to patients in urban areas. A systematic review showed this to be the case for at least lung, colorectal and prostate cancer14. The disparities between urban and rural healthcare are magnified in middle- and low-income countries, and countries with poorly resourced healthcare systems. The reasons underlying this disparity are multifactorial, but access to laboratory medicine and cancer histopathology services are critically important factors15. This raises two questions: Can digital pathology help address a lack of access to diagnostic histopathology? Is digital pathology with remote reporting feasible and useful in these settings?
As with much innovation, digital pathology is an evolution of an earlier technology: telepathology. In the pre-WSI era this allowed transmission of still photographs of regions of interest from a glass slide, or remote robotic control of a light microscope with an attached digital camera16. Still-image telepathology has an unsurprising reduction in diagnostic fidelity compared to WSI as only selected areas are imaged at a reduced range of magnifications. However, still-image diagnosis can still achieve good accuracy17. Training of technical staff to acquire useful images is crucial, which can be challenging in rural and remote settings. The choice between WSI and traditional telepathology is therefore not necessarily clear-cut. Infrastructure may present a major challenge to implementation. The storage and transmission of digital pathology slides are non-trivial problems. Without image compression a single WSI image may be more than 4 GB in size. The required image storage capacity for a laboratory expands rapidly as workload increases. The need to archive scanned slides compounds storage requirements. Sufficient bandwidth is also required to transmit digital slides.
In the study by Kawakami et al, a dedicated digital fibre was used for image transfer7. However, in settings where this isn’t available, slide transmission may be unreliable, nullifying the benefits of slide scanning. In their study of digital pathology in Rwanda, Mpunga et al18 implemented static image acquisition rather than WSI owing to an unreliable internet connection with low bandwidth18. A study in Cameroon described the development of a system where initially still images were taken from a light microscope with an iPhone and digitally transferred to Austria for diagnosis19. When WSI was introduced the time to diagnosis and non-diagnostic images were reduced, indicating an improvement in the system. However, the authors identified an unreliable power supply as an issue when scanning and transferring WSI images. The changing landscape of internet connectivity may provide an opportunity to overcome low bandwidth in rural and remote areas. Up to 90% of internet connectivity in Africa is mobile rather than wired broadband20. As 5G networks replace the existing 3G and 4G infrastructure this enhanced connectivity could facilitate digital slide transmission in local/regional pathology networks or abroad, as required. Together these various challenges represent considerations for implementation of digital pathology (Table 1).
Digital pathology also offers benefits beyond the provision of a diagnostic service. Training and mentorship have been identified as key areas for development in rural and remote pathology. Surveys of laboratory medicine professionals in low- and middle-income countries have identified mentorship as a key aspect of improving pathology services15. Digital pathology can facilitate mentorship and training by various routes. There are thousands of WSI images available online to aid learning as part of histopathology training programs21. This same technology can be adapted to provide course-specific training and WSI incorporated into asynchronous learning programs. Furthermore, WSI images can be annotated to provide granular instruction when this may not be available locally. Many external quality assurance schemes now provide digital slides and are another route to accessing expertise and staying up to date with diagnostic practice. One issue identified with implementing a pathology training program in Zambia was the difficulty of securing time for external clinicians to attend physically to supplement training provided by local doctors22. This program started in 2011, while WSI was still a nascent technology. If implemented today, access to external trainers could be provided digitally, leveraging WSI and videoconferencing.
In addition to widening access to training materials, the digitization of glass slides presents new opportunities in image analysis and deep learning. The past 10 years have seen an explosion of interest in applying deep learning techniques to histology slides to predict survival and response to treatment, and also to better quantify assessment traditionally made by the naked eye such as tumour grade and quantification of protein expression by immunohistochemistry23. These technologies could be enormously useful in the remote and rural healthcare setting, potentially acting as triage for cases that need urgent review or supplementing existing expertise, particularly if local exposure to a certain case type is limited. To date, there is little evidence assessing deep learning and image analysis specifically in rural and remote cancer care. There will be variability between settings but it is important that systems and tools developed in urban settings are also validated for use in less populated environments, as the case mix and system requirements may well be different. Remote environments may have common themes for which pathology services require support (eg frozen section diagnosis), and deep learning adjuncts should be tailored towards these areas.
Digital pathology has the potential to transform rural and remote cancer pathology, providing faster diagnoses, building local expertise through training and mentorship and potentially through computer-aided diagnostics. There are many challenges including infrastructure and the specifics of implementation for each healthcare setting. Rural and remote digital pathology is relatively underrepresented in the literature and future research should be designed with this in mind, including the areas of:
- systematic review of digital pathology in rural and remote health care
- development of deep learning tools for specific rural and remote digital pathology applications
- use of digital pathology in training and mentorship
- analysis of available and projected infrastructure and its ability to support digital pathology.
Table 1: Considerations in implementing a digital pathology system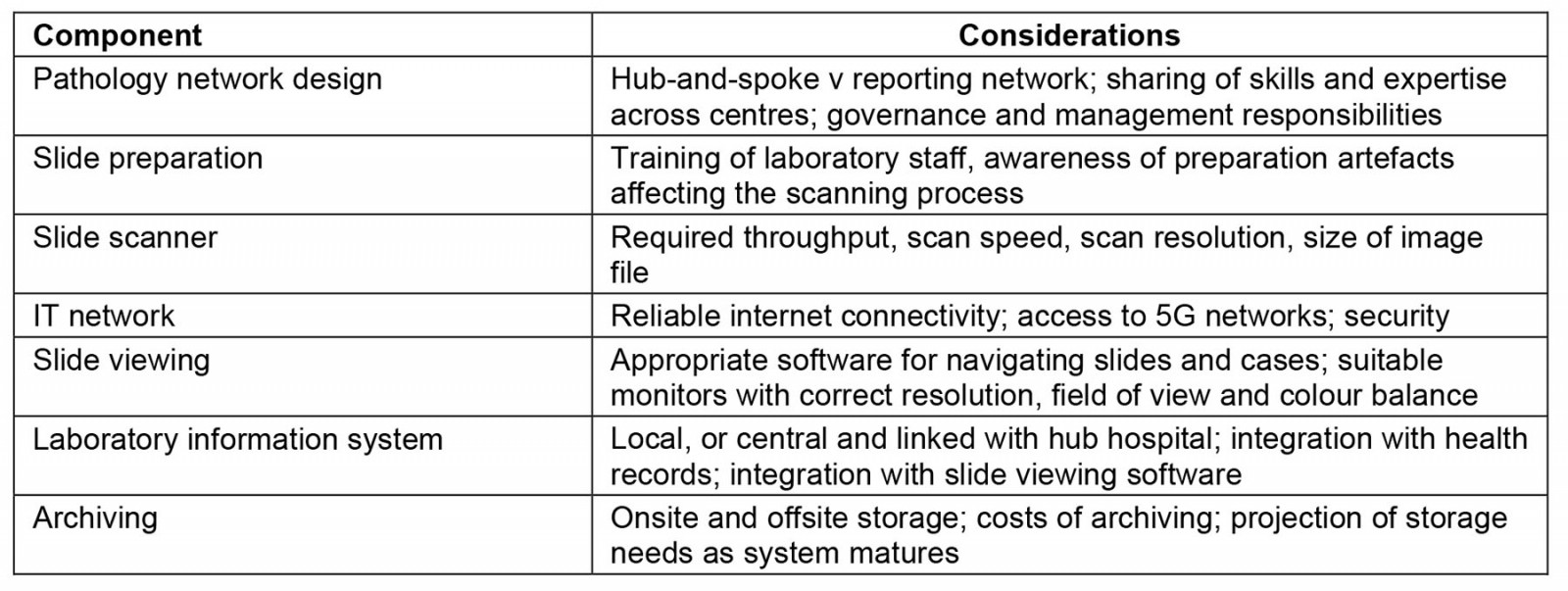
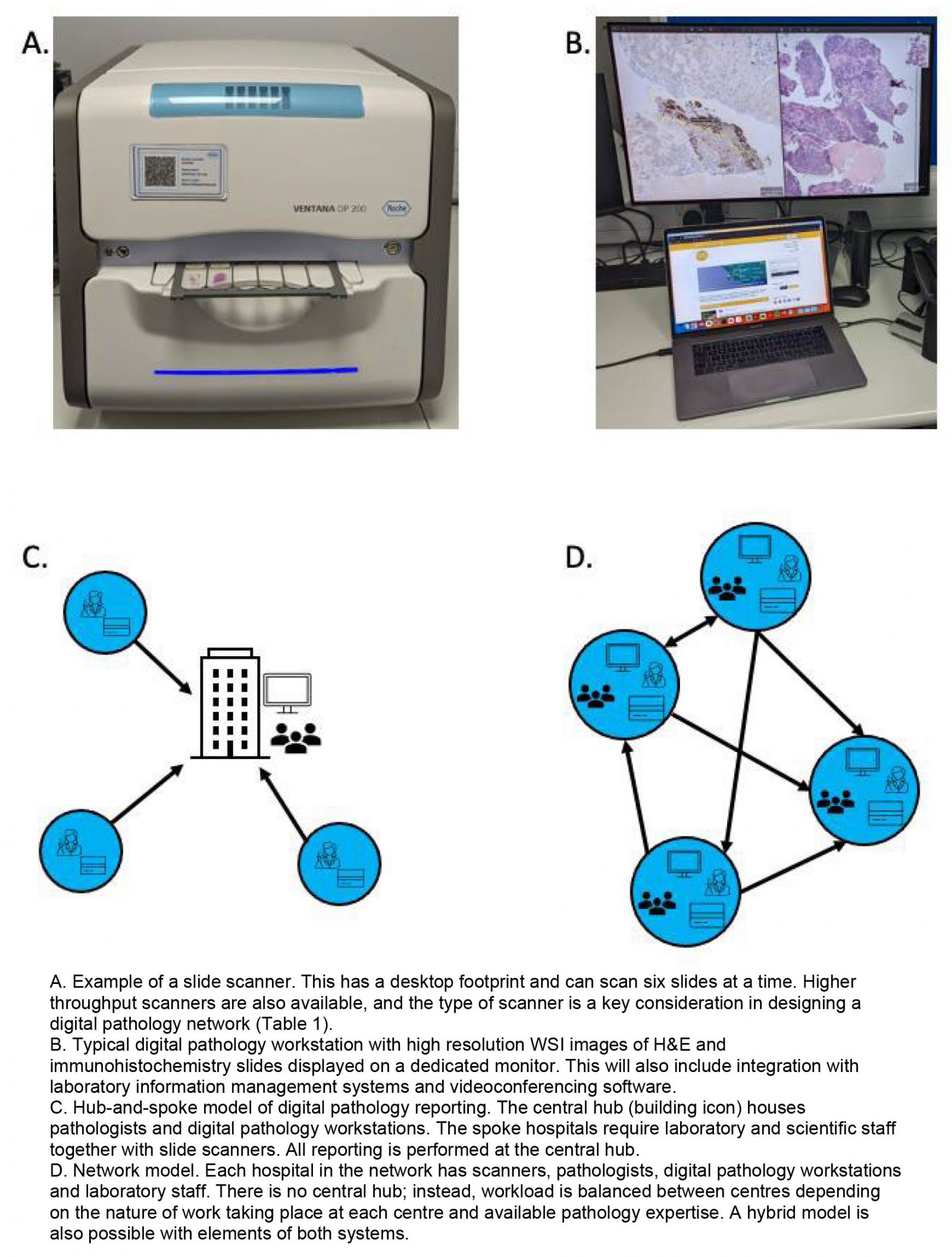 Figure 1: Digital pathology: the hub-and-spoke and networked models.
Figure 1: Digital pathology: the hub-and-spoke and networked models.
