Introduction
Infections requiring hospitalization impose significant costs on healthcare systems worldwide. Many economic reports are derived from readily available volume data including bulk costs and pharmacy supply statistics, which do not necessarily correspond to actual costs of antibiotic and resource utilization, as well as everyday clinical practice1. A recent effort to overcome this obstacle has been made in Greece in relation to community-acquired pneumonia2. This indicated that hospitalization, medication and diagnostic tests mainly drove inpatient costs in cases of community-acquired pneumonia. Data is scarce regarding other sites of infection. We hypothesized that, other than for antibiotic misuse, infection site has a significant impact on antibiotic- and hospital-related cost variability.
Methods
This prospective study was carried out in the medical ward of a tertiary university hospital in Greece between 1 May 2016 and 1 May 2018. Adult patients admitted at the medical ward with established respiratory tract infection (RTI), urinary tract infection (UTI), gastrointestinal infection (GI), skin, soft tissue and bone infection (SSTBI) or primary bacteremia (PB) were included in this study and followed up until discharge. Epidemiological characteristics, assessment of disease severity as per Sepsis-II3 and Sepsis-III definitions4, empiric antibiotic therapy and in-hospital outcome (survival rate and length of stay (LOS)) were recorded. Costs of hospitalization and unit cost of antibiotic regimen were retrieved from a national health system database for Greek hospitals containing data for each International Classification of Disease (ICD-10) code5 and the national formulary respectively, and manually calculated for each patient.
Statistical analyses
All statistical analyses were performed using SPSS for Windows v25 (IBM International; http://www.spss.com).
Continuous variables are presented as mean ± standard deviation, with ranges or with 95% confidence intervals. Categorical variables are presented as proportions of the sample as a whole. The significance of differences between two independent samples for continuous variables was measured with student t-tests or the Mann–Whitney U-test, also depending on the normality of the data distribution (Kolmogorov–Smirnov test results). For comparisons of continuous variables among three or more groups, we used one-way ANOVA or the Kruskal–Wallis test, again depending on the normality of the data distribution. Linear correlations between key variables were tested by Spearman’s and eta correlation coefficients. The strength of each correlation was assessed according to Cohen’s kappa (≥0.5, extremely strong; 0.3–0.49, moderate; ≤0.29, weak). The level of statistical significance was set at p<0.05.
Ethics approval
This study was approved by the University Hospital of Patras ethics committee (number 96/15.04.16) and carried out in accordance with relative regulations, following subjects’ consent.
Results
In total, 629 patients fulfilled inclusion criteria during the study period. Characteristics of patients, infections and antibiotics, costs and outcome are shown in Tables 1 and 2 respectively. Patients with PB and SSTBI generally presented in more severe condition and this reflected in the percentage of patients with a qSOFA sepsis score ≥2 and the percentage of patients with sepsis compared to those with RTIs, UTIs and SSTIs. PB presented the lowest survival rates. The majority of infections were community acquired, except for PB and SSTBI, which were equally divided between hospital and community origin. PB and SSTBI infections presented the longer LOS, similar to increased antibiotic and hospital-associated cost compared to other sites of infection. LOS, antibiotic- and hospital-related costs all significantly differed between infections. Antibiotic- and hospital related costs are strongly related with LOS in all infections (p<0.001 for all sites). Site of infection is moderately related to length of stay (eta value 0.444), antibiotic cost (eta value 0.445) and hospital-related cost (eta value 0.387).
Table 1: Characteristics of patients admitted with bacterial infections to a tertiary university hospital, Greece, May 2016 – May 2018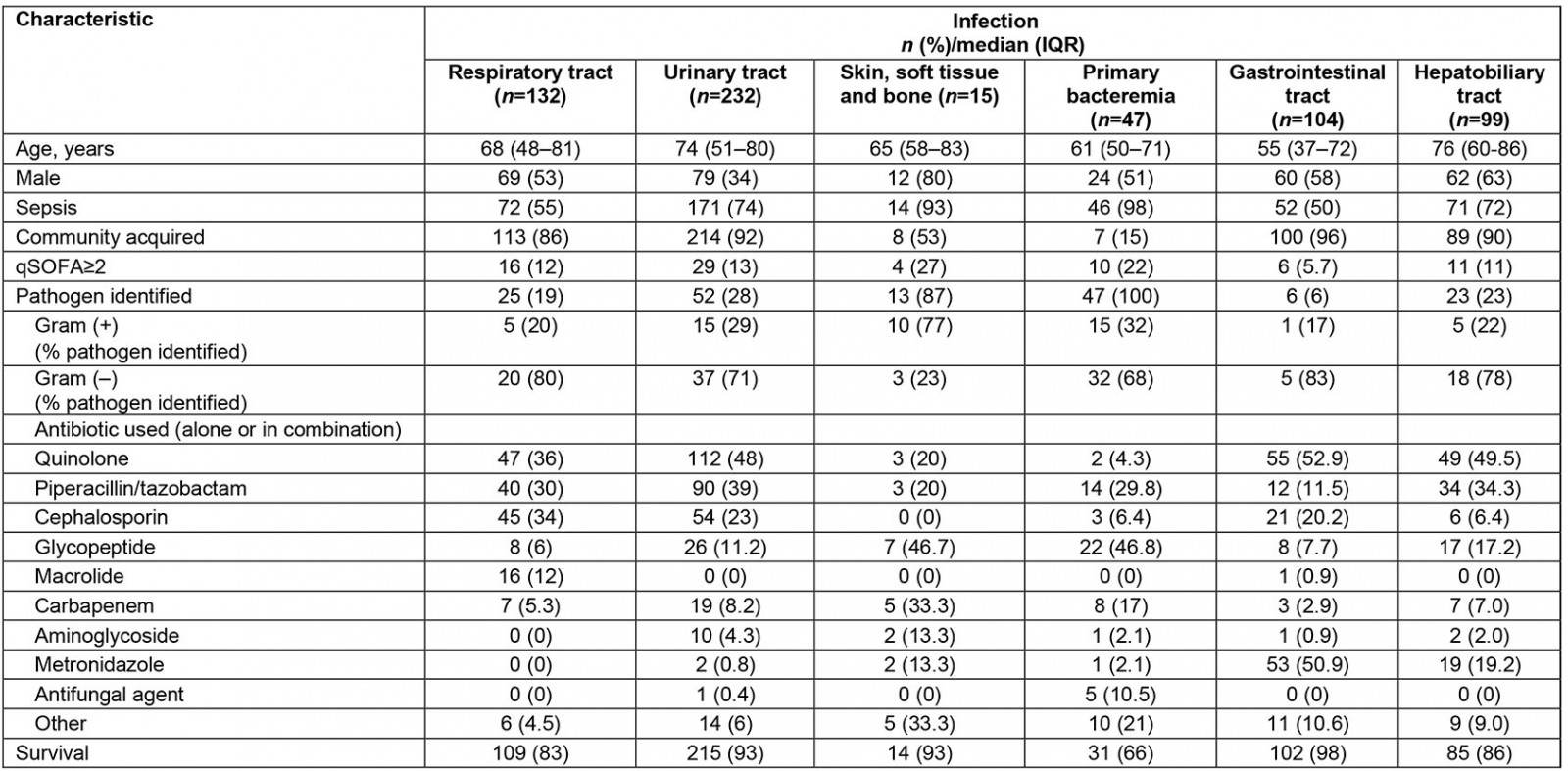
Table 2: Cost and length of stay for patients admitted with bacterial infections to a tertiary university hospital, Greece, May 2016 – May 2018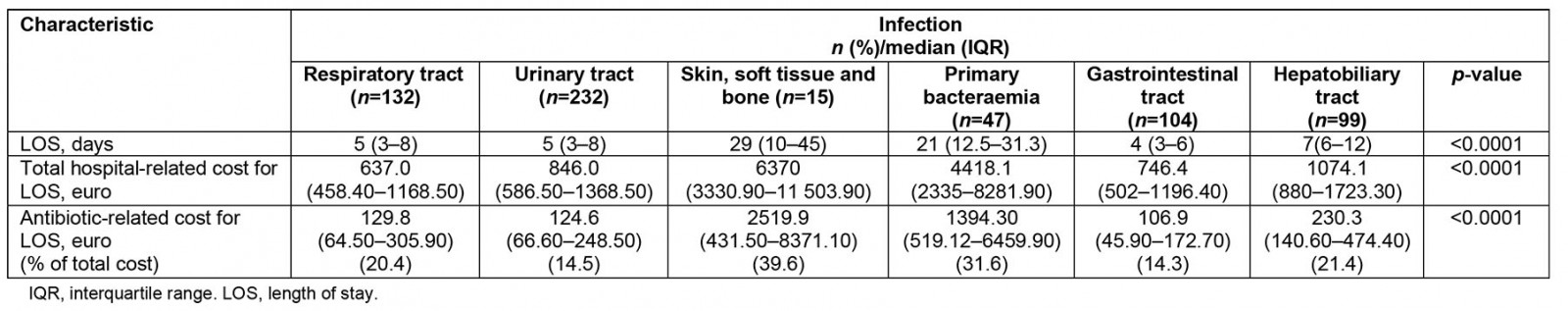
Discussion
We performed a prospective study exploring the healthcare costs in relation to site of infection. We found that antibiotic- and hospital-related costs significantly varied among different infections, and is correlated strongly to LOS and moderately to type of infection. We observed that PB and SSTI appear to be the most costly infections, increasing the average antibiotic cost 10-fold compared to other infections, representing approximately one-third of total hospital-associated related costs.
In the case of SSTI, increased cost seems to be attributed to extended LOS, mostly driven by bone infection requiring long courses of intravenous therapy, despite recent data favouring oral therapy, hence antibiotic misuse1,6. Antibiotic misuse has been previously recorded in the setting of community-acquired pneumonia in Serbia, significantly elevating healthcare related costs7. Similarly, PB requiring specific days of intravenous therapy is responsible for increased LOS, thus increasing cost. However, in the case of PB it seems that a high percentage of hospital-acquired PB with potential drug-resistant pathogens, complicated by secondary infections and demand for multiple rounds of antibiotics, increased the total expenditure8. It is notable that, at the time of this study, dalbavancin, ceftolozane/tazobactam and ceftazidime/avibactam were not available in the host institution.
The costs of antibiotic therapy have been assessed in the past in relation to guideline-adherent appropriateness of initial therapy or use of broad-spectrum antibiotics1,9,10. Absolute costs vary significantly between settings, and this could be attributed to antibiotic production of individual countries, supplies, pharma negotiation and federal policies. Most information is derived from cases of respiratory tract infection2, because no comparative data between infections exist11,12. In all cases, inappropriate empirical antibiotic therapy, which is defined by later pathogen identification or clinical failure, significantly increases LOS (by approximately 2 days), and antibiotic- and hospital-related costs (also observed in our group; unpublished data). The present study found that antibiotic costs seem to represent approximately 15–20% of total hospital costs, in contrast to recent data from US hospital cohorts attributing 3–5% of hospital-associated costs to antibiotics9. Bearing in mind that this study was performed during the financial crisis in Greece, this observation is important.
Even though the hospital in this study is a tertiary university teaching hospital, conforming with international guidelines on antibiotic therapy, one cannot predict variance among doctors’ prescription habits and compliance with stewardship programs13. Hospital administrations should begin to consider the importance of appropriate choice of antibiotic therapy rather than simply the pharmacy budget (eg including solely generic regimens to improve costs). A recent evaluation in Kazakhstan of implementation of drug literacy interventions among healthcare workers showed positive outcomes for antibacterial consumption, hence decreasing associated costs14. Alternative interventions that require added outlays to improve mortality should be weighed against competing demands15. Infectious disease specialist consultation strategies and strict implementation of local stewardship protocols have had variable results in the past16. Specialist nursing or self-administered intravenous therapy in an outpatient setting could prove cost effective for both short- and long-term infections17.
Potential solutions should be explored in the context of ageing populations and their needs, and take advantage of innovation in digital health to allow for self-care and greater independence and cost-effectiveness, while weighing this against limited healthcare expenditure15,18. Also to be considered are needs and realities created by the COVID-19 pandemic, which has overstretched healthcare expenditure through irrational and increased use of antibiotics both in wards and intensive care units19,20. Reallocated priorities have held back antibiotic surveillance, possibly placing us in the midst of rising antimicrobial resistance in the years following the pandemic21.
Our study has inherent limitations. Although it spanned a 2-year period, blunting potential variations in time (such as antibiotic class cycling), it remains a single-centre study. Variable antimicrobial resistance environments may complicate and elongate antibiotic management, thus adding costs. Also, our study was designed to record in-hospital costs of infection management. Costs following discharge were beyond the scope of this study. A larger multi-centre study would allow for a multivariable analysis to show other potential independent predictors of cost variability, including site of infection, with greater certainty. Beyond these limitations, this study is one of the first studies to explore real-world antibiotic and hospitalization costs in a Greek setting, examining different sites of infection.
Conclusion
Healthcare-related costs vary substantially depending on site of infection. Information about real-life costs can drive best decisions and help us reduce healthcare expenditures. This was the first study to attain real-world cost data in Greece, pertaining to different sites of infection, providing a greater picture of antibiotic use while suggesting integrated curative and preventive interventions on how this can be moderated in a setting of stringent budget and high antimicrobial resistance.
References
You might also be interested in:
2008 - The future, our rural populations and climate change - a special issue of Rural and Remote Health

